- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
06. May 2018
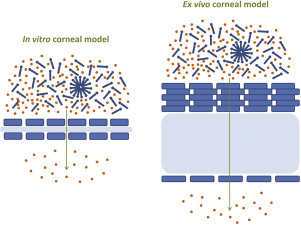
The objective of this study was to systematically investigate the effects of surface active ophthalmic excipients on the corneal permeation of ophthalmic drugs using in vitro(HCE-T cell-based model) and ex vivo (freshly excised porcine cornea) models. The permeation of four ophthalmic drugs (i.e., timolol maleate, chloramphenicol, diclofenac sodium and dexamethasone) across in vitro and ex vivocorneal models was evaluated in the absence and presence of four commonly used surface active...
18. November 2017
The objective of the present study was to develop rectal mucoadhesive hydrogels loaded with Tolmetin Sodium, a non-steroidal anti-inflammatory drug, for prolonged duration of action and increased bioavailability. Fourteen formulae were prepared with different types and concentrations of polymers as hydroxypropylmethyl cellulose, hydroxylethyl cellulose, carboxymethyl cellulose and sodium alginate. Each formulation contain Tolmetin Sodium equivalent to 5% w/w active drug. The effect of the...
04. September 2017
Abstract Recently, different technologies have been used to transform active pharmaceutical ingredients (APIs) into new dosage forms. Engineered drug delivery systems may modify biopharmaceutical properties of the API achieving either immediate or delayed release according to specific therapeutic needs. Particularly, preprogrammed release of oral formulations delivering the drug at expected times may be useful in chronotherapy of early morning pathologies. The conventional approach when dealing...
04. April 2017
Abstract The objective of this study was to develop Esomeprazole solid dosage form as minitablets. The effect of coating thickness and percentage of fast disintegrants on the in vitro and in vivo performance of minitablets were studied. Two formulae (A1&B1) of the same core composition with different coat thickness were prepared initially. The in vivo study of A1 and B1 versus the originator revealed that their rate of dissolution was not enough to achieve bioequivalence with respect to...
15. March 2017
Abstract The present study was designed to prepare and compare bio-adhesive pellets of panax notoginseng saponins (PNS) with hydroxy propyl methyl cellulose (HPMC), chitosan, and chitosan : carbomer, explore the influence of different bio-adhesive materials on pharmacokinetics behaviors of PNSbio-adhesive pellets, and evaluate the correlation between in vivo absorption and in vitro release (IVIVC). In order to predict the in vivo concentration-time profile by the in vitro release data of...
08. March 2017
Abstract This study was designed to develop a once-daily controlled-release matrix tablet of aceclofenac 200 mg (AFC-CR) with dual release characteristics and to investigate the role of an alkalizer in enhancing drug solubility and reducing the occurrence of gastroduodenal mucosal lesions. Two formulation approaches were employed, namely a monolithic matrix tablet and a bilayered tablet. In vitro dissolution studies of AFC-CR tablets were carried out in simulated intestinal fluid (pH 6.8...
03. March 2017
Abstract The study aimed to characterise the mechanism of release and absorption of Basmisanil, a biopharmaceutics classification system (BCS) class 2 compound, from immediate-release formulations via mechanistic absorption modelling, dissolution testing, and Raman imaging. An oral absorption model was developed in GastroPlus® and verified with single-dose pharmacokinetic data in humans. The properties and drug release behaviour of different oral Basmisanil formulations were characterised via...
27. February 2017
Abstract: The main challenges facing efforts to prevent the transmission of human immunodeficiency virus (HIV) are the lack of access to sexual education services and sexual violence against young women and girls. Vaginal formulations for the prevention of sexually transmitted infections are currently gaining importance in drug development. Vaginal mucoadhesive tablets can be developed by including natural polymers that have good binding capacity with mucosal tissues, such as chitosan or guar...
10. February 2017
Purpose To develop a model linking in vitro and in vivo erosion of extended release tablets under fasting and postprandial status. Methods A nonlinear mixed-effects model was developed from the in vitro erosion profiles of four hydroxypropyl methylcellulose (HPMC) matrix tablets studied under a range of experimental conditions. The model was used to predict in vivo erosion of the HPMC matrix tablets in different locations of the gastrointestinal tract, determined by magnetic marker monitoring....
09. August 2016
Abstract Context: Considering that bitter taste of drugs incorporated in orally disintegrating tablets (ODTs) can be the main reason for avoiding drug therapy, it is of the utmost importance to achieve successful taste-masking. The evaluation of taste-masking effectiveness is still a major challenge. Objective: The objective of this study was to mask bitter taste of the selected model drugs by drug particle coating with Eudragit® E PO, as well as to evaluate taste-masking effectiveness of...