- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
31. August 2018
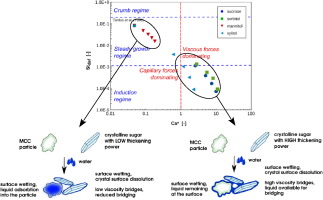
Sugars and sweeteners are common ingredients in foods and also in pharmaceutical industries. They are soluble and sticky ingredients and their processing in granulators may be difficult since they can easily adhere to mixer walls or leadto uncontrolled granule growth. The purpose of this research was to evaluate the feasibility of the high shear wet granulation process and to find the optimal amount of binder by studying the granulation performances of four sugars: mannitol, sorbitol, xylitol...
23. July 2018
There are a multitude of sweeteners available to the pharmaceutical scientist. Sweeteners may be broadly grouped into two categories – nutritive and non-nutritive. Nutritive sweeteners delivercalories and as their name suggests, non-nutritive do not. Non-nutritive sweeteners can be further characterized as bulk (sugar alcohols) and high intensity (artificial). Some excipients that are sweet are often incorrectly listed in publications as “sweeteners.” These are excipients used primarily...
17. November 2016
Abstract Orally Disintegrating Tablets (ODT) have the advantages of both solid dosage form specially the stability and ease of handling and liquid dosage forms including ease of swallowing and pre-gastric absorption. We focused on taste masking and formulation of ranitidine ODT which disintegrates rapidly in the mouth within 60 sec using super-disintegrants, special polymers, water soluble and even insoluble excipients, sweeteners and essence. Various formulations were designed and made in four...
16. November 2016
Orally Disintegrating Tablets (ODT) have the advantages of both solid dosage form specially the stability and ease of handling and liquid dosage forms including ease of swallowing and pre-gastric absorption. We focused on taste masking and formulation of ranitidine ODT which disintegrates rapidly in the mouth within 60 sec using super-disintegrants, special polymers, water soluble and even insoluble excipients, sweeteners and essence. Various formulations were designed and made in four series....
12. November 2015
08. June 2015
The STEP stands for Safety and Toxicity of Excipients for Paediatrics. The Safety and Toxicity of Excipients for Paediatrics (STEP) Database is a user-designed resource developed by European Paediatric Formulation Initiative (EuPFI) and United States Paediatric Formulation Initiative (USPFI) in collaboration for storage and rapid/effortless access to the safety and toxicological data of excipients that is scattered over various sources and presents it in one freely accessible source. It is a...