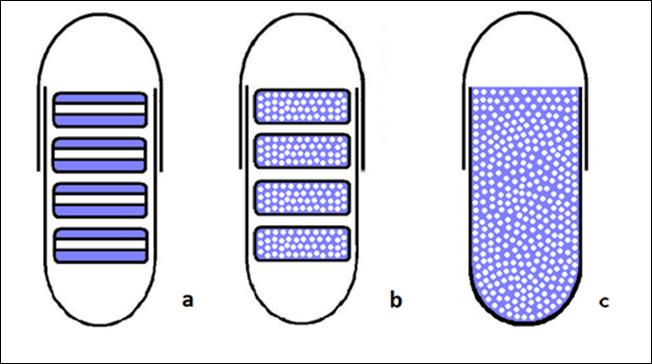
In pharmaceutical research, new dosage forms that modify drug release are useful for circadian rhythm related diseases. The aim of this investigation is to examine the release behavior of multiple-unit modified-release formulations (‘tablets in capsule’) of theophylline. Mono-layered and three-layered minitablets, filled into capsules, were prepared using theophylline and dextran or pectin, as excipients. Their release behavior was compared with respect to powder filled capsules and the commercially available Theodur® 200 mg tablets. Dissolution tests were performed in three pH media (1.5, 7.4 and 6.0) in the presence and absence of the enzyme Pectinex® Ultra SP-L solution, which degrades polysaccharides. The results indicate that enteric-coated capsules showed no drug release, in the first two hours in the acidic media. Dextran produces a thicker gelled layer than pectin and therefore, dextran based ‘tablets in capsules’ systems are suitable as a model extended release formulation. The drug is released from 3-layered minitablets at a slower rate compared to matrix minitablets. The product, Pectinex® Ultra SP-L, was found to give more accurate dissolution results when used as an additive to the media, mimicking the large intestine/colon area especially when the formulations target this region.
Pharm Pharmacol Int J 5(2): 00115. DOI: 10.15406/ppij.2017.05.00115
Recommended for you
- Reformulation of Tablets to resolve sticking & picking issues
- Captisol - modified cyclodextin for improved solubility, stability, bioavailability
- Excipient DMF List
