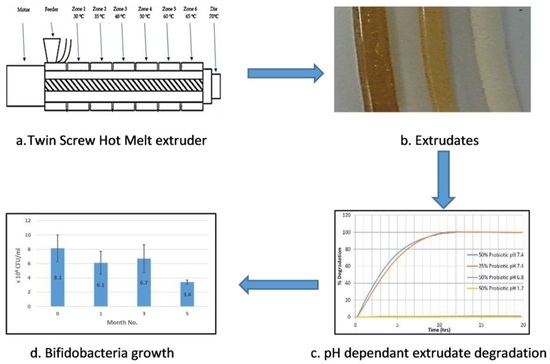
Hot melt extrusion (HME) is considered an efficient technique in developing solid molecular dispersions, and has been demonstrated to provide sustained, modified and targeted drug delivery resulting in improved bioavailability. However, most commercial enteric or pH-responsive polymers are relatively difficult to process or have high Glass Transition Temperature (Tg) values, making their use with temperature-sensitive drugs, probiotics or biologics not viable. Shellac is a natural thermoplastic, and after a review of current literature on the pharmaceutical HME process, a possible gap in the knowledge of the use of shellac to produce dosage forms by means of HME was identified. This work explores the possibility of SSB® 55 pharmaceutical-grade shellac as a melt-extrudable encapsulation polymer to entrap freeze-dried probiotic powder and to determine bacterial cell viability post-processing. Well-defined strands were produced from the physical mixture of shellac and Biocare®Bifidobacterium Probiotic. FTIR clarified that there are no significant interactions between the probiotic and polymer. All of the samples demonstrated less than 5% degradation over 24 h at pH of both 1.2 and 6.8. At pH 7.4, both loaded samples gave a similar dissolution trend with complete degradation achieved after 10–11 h. Following five-month storage, 57.8% reduction in viability was observed.