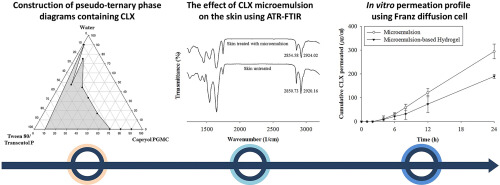
The aims of this study are to prepare and characterize a microemulsion-based hydrogel containing celecoxib (CLX) for transdermal delivery. Pseudo-ternary phase diagrams containing 0.74% (w/w) CLX were constructed usingawater titration method. The mixture exhibiting the largest area of the microemulsionregion was selected. The effect of the ratio of oil to themixtureof surfactant and cosurfactant (O/Smix) on in vitro skin permeation was investigated. Attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) was employed to investigate the effects of the CLX microemulsion on skin. Rheological measurementswereperformed, and in vitro skin permeation of the CLX microemulsion-based hydrogel was tested. Based on pseudo-ternary phase diagrams, the Capryol PGMC-Tween 80-Transcutol P mixturewasselected because it had the largest microemulsion area. A CLX microemulsion with O/Smix ratio of 3/7 was chosen because it had the greatest in vitro skin permeation. ATR-FTIR demonstrated that CLX microemulsion led to a transition in the alkyl chain of SC lipids and permeated hairless mouse dorsal skin. TheCLXmicroemulsion-based hydrogel was elastic and suitable for application to skin. The incorporation of a gelling agent controlled the drug release of CLX, implying improved therapeutic effects of CLX for arthritis.