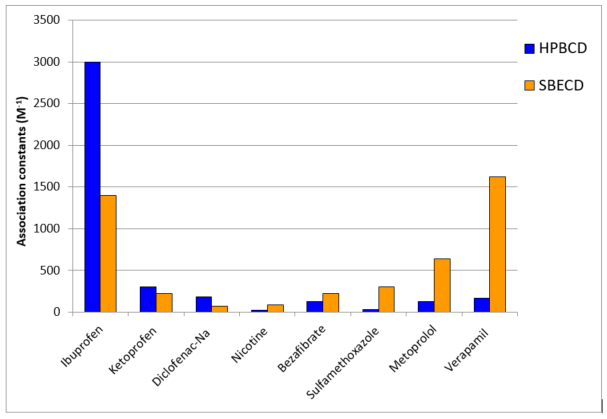
A comparison of Hydroxypropyl and Sulfobutyl Betadex
As a result of thorough development work, CycloLab Ltd (Budapest, Hungary) has established and validated the production of USP-complying Betadex Sulfobutyl Ether Sodium formulation excipient (SBECD, DexolveTM) in 2008. This cyclodextrin (CD) derivative is the main component of several
commercial drug formulations worldwide acting as a solubility and stability enhancer. SBECD is one of the two parenterally applicable cyclodextrin derivatives besides Hydroxypropyl Betadex (HPBCD), nevertheless both of these substances are just as suitable in drug products of various administration
routes. In order to highlight the special properties of SBECD it is worth comparing these two injectable composite substances. Both CD derivatives are resultant of substituting neat beta-cyclodextrin at maximum 21 possible hydroxyl moieties in a random manner, i.e. both end products are complicated mixtures of modified CD species of different average degree of substitution (DS) as well as vast number of regioisomers. However, it is possible to characterize the distribution of such species which is an important quality parameter of both composites. Thorough toxicological safety studies were performed for both HPBCD nd SBECD meeting specific compositional profiles, which are by now standardized regulatory wise to overcome the said variability. HPBCD has a monograph in both United States Pharmacopoeia (USP) and European Pharmacopoeia EP), while SBECD is listed only in USP.