connecting biology, physics and digital for the benefit of human wellbeing.
If you look over your shoulder you can connect the dots. If you look at the past you turn your back to the future. This provides an interesting paradigm for Industry 4.0 / Pharma 4.0. Is it a hype that our regulated industry should turn its back to? Or can we connect the dots to a Pharma 4.0 future?
We invited Hans Heesakkers of Circuition Life Science Consultants to the Pharma lunch of the Swiss Academy of Pharmaceutical Sciences (SAPhS) in Basel on March 2nd, 2018.
Hans Heesakkers

Hans brings an experience of 30 years in the pharmaceutical industry. About half of that as a Consultant innovating R&D and Supply chain.
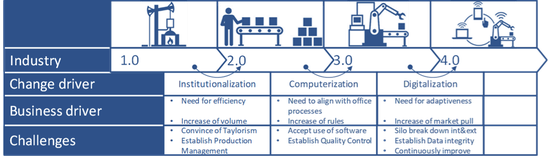
He explains every company aligns the different segments of their operating model with the state of scientific progress and market demands. In history there were at several moment disruptive new technology inventions that created a leap in the development of operating models. The World Economic Forum identified “Digitalization” as the driver behind the 4th industrial revolution. Governments of industrialized countries are actively pushing adoption of the new technological abilities!
Pharma 4.0
During the presentation of many interesting slides we were made aware that although technology might have triggered this revolution, Pharma 4.0 is about changing the industry’s operating model with regards to processes, integration, workforce and quality management. Only if all value chains in our organization are changed on all those segments, companies can keep up with the pace of adaptiveness needed to fulfill medical demand.
Life science organizations often feel they are inert to such developments due to their regulated nature. That is a misperception. If you add up the current regulatory developments, you can see regulators are paving the road for Pharma 4.0. Think about developments like “Data Integrity”, “IDMP”, ICH Q10 &12 and many more.
If you consider the different value chains in the industry as part of a value network, then the first industrial revolution was about information exchange within an operation, the second was about information exchange within a process, the third about information exchange within a value chain and now the fourth is about exchanging across the value network. This means that data needs to travel with integrity and organizations need to organize for adaptive processes, less and ergonomically ideal human-machine interfaces and continuous quality improvement.
PRO Solution by Circuition
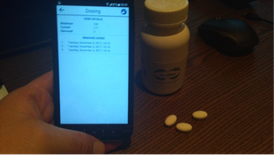
Hans concluded by demonstrating the smart PRO solution of Circuition. In the 4th industrial revolution, life science companies will engage more closely with patients using mobile technology. The solution provides an electronic mobile patient diary in which a patient can log his fitness, treatment effectiveness and unexpected adverse events. Research of the WHO learns that “treatment adherence” and “temperature control” have the largest effect on treatment outcomes. (More than 50% of chronic patients do not adhere to their treatment). The demonstrated smart bottles and blisters monitor their own temperature and the number of tablets that were dispensed from it. By just approaching the medication packs with a smart phone, the patient is shown how “compliant” he is with his treatment regimen and if there are any worrying temperature excursions. After allowance of the patient the data was passed on to a database for the patient’s medical care providers.

If the very positive and interactive response of the audience is a measure for the success of Pharma 4.0. then I recommend we all learn about this.
The presentation on Pharma 4.0 can be downloaded here
The SAPhS PharmaLunch is a monthly event in Basel - more information on http://saphw.ch/en