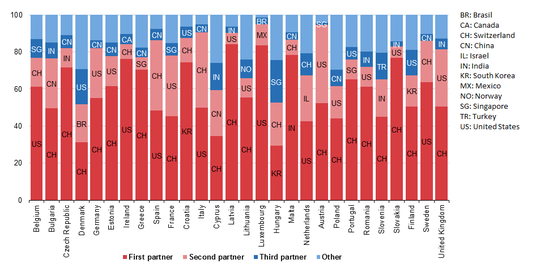
The European Union is a large importer of medicinal and pharmaceutical products. Its main trading partners are the United States and Switzerland but also Brazil, China, Israel, India, South Korea, Mexico, Norway, Singapore and Turkey showed strong presence in 2016.
Sponsored content
Challenges of a EU Import
The major challenges for import activities into the EU are related to
- Customs clearance (how to determine appropriate customs procedures)
- Taxes /VAT (how to legally avoid import turnover taxes, how to optimize cash situation)
- Legal aspects (how to deal with import permissions, how to get batches certified by a Qualified Person and released to the EU market)
Target for deliveries to the EU: Implement a FULL SERVICE situation!
- Get the legally required activities regarding EU import, batch certification and EU Release of the drug products properly executed
- Get the handling of import turnover taxes and customs duties optimized, secure tax-free deliveries to the final EU recipients, optimize your cash situation, use a fiscal representative
- Get appropriate customs procedures established for each individual situation
- Take advantage of a highly professional logistics service organization providing you with the needed logistics services
Continue reading in the following pdf file: