- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
13. February 2017
Abstract In order to save time and resources in early drug development, in vitro methods that correctly predict the formulation effect on oral drug absorption are necessary. The aim of this study was to 1) evaluate various BCS class II drug formulations with in vitro methods and in vivo in order to 2) determine which in vitro method best correlates with the in vivo results. Clarithromycin served as model compound in formulations with different particle sizes and content of excipients. The...
07. June 2016
Purpose Currently, the FDA allows biowaivers for Class I (high solubility and high permeability) and Class III (high solubility and low permeability) compounds of the Biopharmaceutics Classification System (BCS). Scientific evidence should be provided to support biowaivers for BCS Class I and Class III (high solubility and low permeability) compounds.
02. May 2016
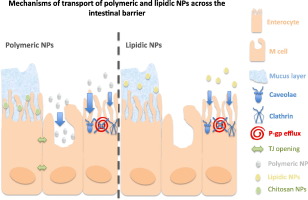
Unraveling the mechanisms of nanoparticle transport across the intestinal barrier is essential for designing more efficient nanoparticles for oral administration. The physicochemical parameters of the nanoparticles (e.g., size, surface charge, chemical composition) dictate nanoparticle fate across the intestinal barrier. This review aims to address the most important findings regarding polymeric and lipidic nanoparticle transport across the intestinal barrier, including the evaluation of...
17. December 2015
The chemotherapeutic drug substance doxorubicin has been reported to be a substrate of P-gp, which induces a barrier for oral administration and leads to a bioavailability of 3% in male Sprague Dawley rats. Literature studies have reported increased transport of P-pg substrates, like digoxin, when co-administered with P-gp inhibitors (non-ionic surfactants) in vitro and in vivo. More