- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
24. November 2017
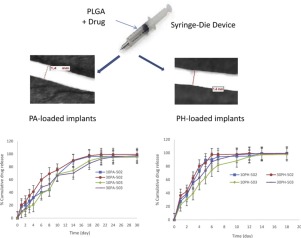
To avoid frequent drug administration, PLGA-based monolithic filament-shaped implants were prepared. In this study, the effect of different formulation variables was studied, namely: type of PLGA (PLGA 502 and PLGA 503), type of drug (the lipophilic Prednisolone acetate, PA and the hydrophilic Propranolol Hydrochloride, PH) and drug loading (10 and 30% w/w). PLGA 503-based implants showed a lower water uptake, lower mass loss and erosion, slower drug release, and better mechanical properties and
28. June 2017
The use of ammonia methacrylate copolymer as a carrier for the preparation of oral controlled-release matrix tablets containing water-insoluble drugs and investigating the effect of curing conditions.
28. April 2017
The rising demand for pharmaceutical particles with tailored physico-chemical properties has opened new markets for the spray drying technology especially for solubility enhancement, improvement of inhalation medicines and stabilization of biopharmaceuticals. Despite this, the literature on spray drying is scattered and often does not address the fundamental principles underpinning the robust development of pharmaceutical products. It is therefore necessary to present a clearer picture of the fi
11. January 2017
Purpose The objectives of this study were to develop once-a-day oral controlled-release tablets of quetiapine fumarate (QF) and to determine the effect of polymer type, viscosity grade, polymer ratio, and polymer rheological properties on the rate of QF release from hydroxypropyl methylcellulose (HPMC) matrix tablets. More
08. November 2016
Abstract The aim of this study was to prepare solid dispersion of nimodipine (NMD) by solvent evaporation method to overcome the poor water solubility of NMD, and to formulate controlled-release (CR) tablets containing the NMD solid dispersion. NMD is a dihydropyridine calcium-channel antagonist that is practically insoluble in water. Owing to the short half-life of the drug in the plasma, NMD immediate-release tablets should be administered frequently for the treatment and prevention of...
18. October 2016
Abstract The present study focuses on the development of dry powders for inhalation as adjuvant chemotherapy in lung cancer treatment. Cisplatin was chosen as a potential candidate for a local treatment as it remains the main platinum component used in conventional chemotherapies, despite its high and cumulative systemic toxicities. Bulk cisplatin was reduced to submicron sizes using high-pressure homogenization, mixed with a solubilized lipid and/or PEGylated component and then spray-dried to...
09. August 2016
The importance of providing safe and effective delayed- and extended-release oral formulations that can replace products requiring multiple administrations has been continually cited as an area in need of improvement for pharmaceutical companies. Such controlled release challenges become especially critical when they must be adapted for paediatrics, those suffering from dysphagia, or patients with specific dosage administration limitations. More often than not, lack of palatability and...
22. July 2016
Though the number of approved controlled-release powder formats is modest, a rising number of pharmaceutical companies and manufacturing organisations are incorporating controlled-release powder manufacturing to their portfolios to address the growing dosage form problem for paediatric and geriatric patients. Cory Berkland, PhD, and Nathan Dormer, PhD, from Orbis Biosciences look at what this delivery system can offer. Link to ONdrugDelivery Magazine
27. September 2015
Prilling represents an innovative technology for continuous particle processing and has been intensively used for the production of urea pellets. The process converts a molten liquid into droplets followed by cooling of the droplets in a prilling tower, generating solid spherical particles with uniform size distribution. More
22. June 2015
In vitro drug release from well-defined particle-size fractions of the mesoporous magnesium carbonate material Upsalite® was investigated in detail using ibuprofen, a biopharmaceutics classification system class II drug, as the model compound. The weight of loaded drug corresponded to 30% of the weight of the carrier and the pores were filled to approximately 80%. More