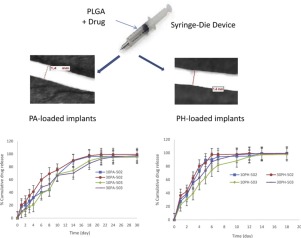
To avoid frequent drug administration, PLGA-based monolithic filament-shaped implants were prepared. In this study, the effect of different formulation variables was studied, namely: type of PLGA (PLGA 502 and PLGA 503), type of drug (the lipophilic Prednisolone acetate, PA and the hydrophilic Propranolol Hydrochloride, PH) and drug loading (10 and 30% w/w). PLGA 503-based implants showed a lower water uptake, lower mass loss and erosion, slower drug release, and better mechanical properties and elasticity (P < 0.05) compared to the corresponding PLGA 502-based implants. PH-loaded implants showed a faster swelling and degradation as well as drug release (P < 0.05) compared to PA-loaded implants; the former attained almost complete drug release after about 18 days, while the latter attained it after about 30 days. All the implants followed a zero-order kinetic pattern suitable for a controlled drug release. Characterization was done using SEM and DSC. This study proved the potential tailoring of the properties of PLGA-implants, prepared by hot-melt extrusion (HME), based on some formulation variables.
Recommended for you