- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
12. April 2018
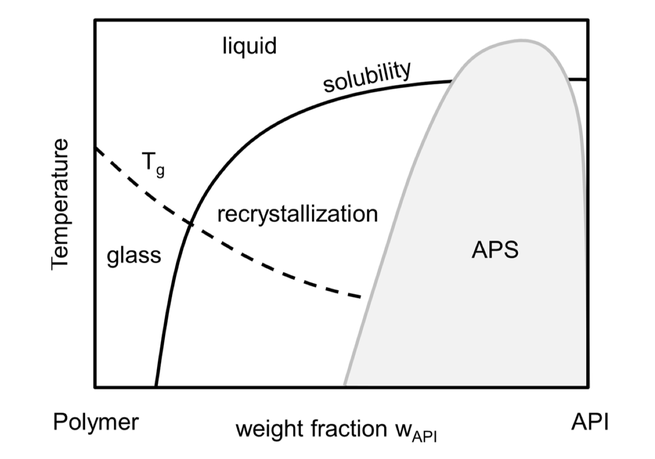
The long-term stability of pharmaceutical formulations of poorly-soluble drugs in polymers determines their bioavailability and therapeutic applicability. However, these formulations do not only often tend to crystallize during storage, but also tend to undergo unwanted amorphous-amorphous phase separations (APS). Whereas the crystallization behavior of APIs in polymers has been measured and modeled during the last years, the APS phenomenon is still poorly understood. In this study, the...
11. April 2018
The use of various harmful organic solvents for microparticle formulations is still widespread. Here, an alternative low toxicity solvent (propylene carbonate; PC) is proposed for the preparation of poly(lactic-co-glycolic-acid) (PLGA) microparticles. Based on the classical emulsification-solvent extraction methodology, the use of PC offers the unique advantage of an additional solvent extraction step using hydrolytic solvent cleavage during microparticle preparation. Spherical, rough-surfaced...
26. March 2018
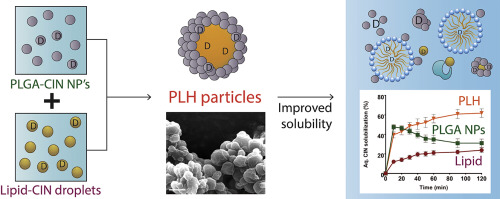
A novel hybrid microparticulate system composed of poly(lactic-co-glycolic) acid (PLGA) nanoparticles and submicron medium-chain triglyceride (MCT) droplets was fabricated to overcome the pH-dependent solubility and precipitation challenges associated with a model poorly water-soluble weak base, cinnarizine (CIN). Molecular CIN was confined within both the lipid and polymer phase of PLGA-lipid hybrid (PLH) and PLGA-lipid-mannitol hybrid (PLMH) particles, which offered significant...
26. January 2018
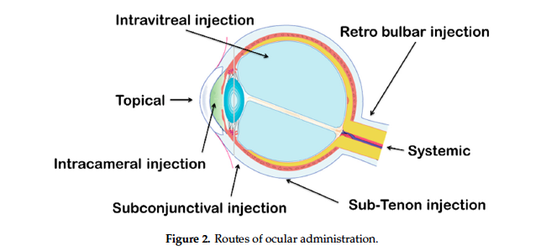
The last fifty years, ophthalmic drug delivery research has made much progress,
challenging scientists about the advantages and limitations of this drug delivery approach. Topical eye
drops are the most commonly used formulation in ocular drug delivery. Despite the good tolerance
for patients, this topical administration is only focus on the anterior ocular diseases and had a high
precorneal loss of drugs due to the tears production and ocular barriers. Antibiotics are popularly
used in solution
24. November 2017
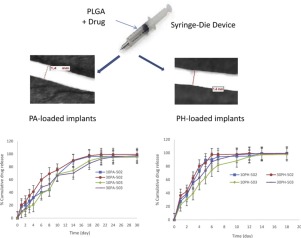
To avoid frequent drug administration, PLGA-based monolithic filament-shaped implants were prepared. In this study, the effect of different formulation variables was studied, namely: type of PLGA (PLGA 502 and PLGA 503), type of drug (the lipophilic Prednisolone acetate, PA and the hydrophilic Propranolol Hydrochloride, PH) and drug loading (10 and 30% w/w). PLGA 503-based implants showed a lower water uptake, lower mass loss and erosion, slower drug release, and better mechanical properties and
31. October 2017
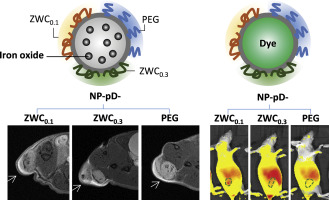
For polymeric nanoparticles (NPs) to deliver more drugs to tumors than free drug solution, it is critical that the NPs establish interactions with tumor cells and avoid removal from the tumors. Since traditional polyethylene glycol (PEG) surface layer interferes with the cell-NP interaction in tumors, we used a water-soluble and blood-compatible chitosan derivative called zwitterionic chitosan (ZWC) as an alternative surface coating for poly(lactic-co-glycolic acid) (PLGA) NPs.
14. August 2017
The porous PLGA particles for pulmonary delivery were prepared.
The shape factor should not be determined only by the outline of particles.
The particles had specific internal structures with unusual aerodynamic performances.
10. May 2017
Colloidal drug delivery systems often face physical and chemical instability as well as challenges with directed delivery. In order to overcome these challenges the colloidal formulations can be processed into microparticulate form (nanoembedded microparticles (NEMs)).
06. March 2017
Abstract We applied the Quality by Design (QbD) approach to the development of poly(lactic-co-glycolic acid) (PLGA) nanoparticle formulations encapsulating triamcinolone acetonide, and the critical process parameters (CPPs) were identified to clarify the correlations between critical quality attributes and CPPs. Quality risk management was performed by using an Ishikawa diagram and experiments with a fractional factorial design (ANOVA). The CPPs for particle size were PLGA concentration and...
17. February 2017
Abstract Dapsone and clofazimine is a known therapeutically effective combination against various Mycobacterium species. However, anti-tubercular effect of this combination is limited due to the low aqueous solubility of clofazimine and production of toxic metabolites after first pass metabolism of dapsone following oral route of administration. Therefore, the aim of this investigation was to develop poly-(lactide-co-glycolic acid) nanoparticles (DCNPS) of dapsone and clofazimine. Nanoparticles...