- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
20. February 2018
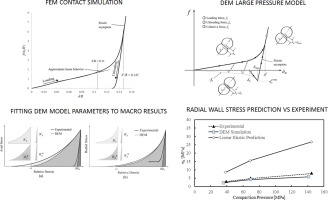
Abstract The mechanical behavior of powders during die compaction was investigated using the discrete element method (DEM), in which powders are modeled as discrete particles with elastoplastic material behavior. A new adhesive elastoplastic contact model that describes the force displacement behavior of contacting particles subjected to high confining conditions was introduced and implemented in DEM simulations of die compaction and uniaxial tension. The objectives of these simulations were:...
13. February 2018
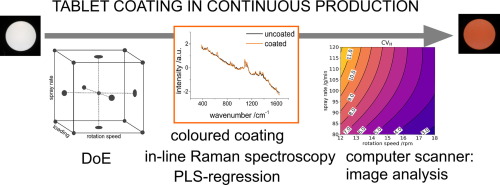
The aim of the study was to optimize a tablet coating process for a continuous manufacturing line. High throughputs should be achieved while inter-tablet coating variability should be as small as possible. Drug-free cores were coated with a colored suspension. All processes were monitored in-line with Raman spectroscopy. A statistical design of experiment was performed to find optimum process parameters.
31. October 2017
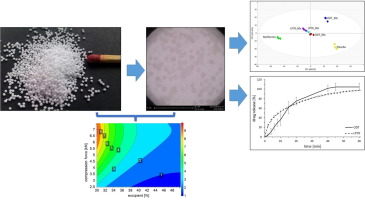
Compaction of multiparticulates into tablets, particularly into orodispersible tablets (ODTs), is challenging. The compression of pellets, made by solid lipid extrusion/spheronization processes, presents peculiar difficulties since solid lipids usually soften or melt at relatively low temperature ranges and due to applied mechanical forces.
31. August 2017
Systematic Design of Experiment (DoE) approach.
Simultaneous analysis of the effect of process and formulation factors.
Studying molecular level interactions via FTIR and SSNMR analyses.
16. May 2017
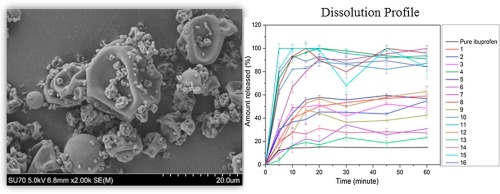
Abstract In this study, a Quality by Design (QbD) approach was used to identify the effect of formulation parameters in a twin screw wet extrusion granulation process for the manufacturing of ibuprofen (IBU) granules with increased dissolution rates. A fractional factorial Design of Experiment (DoE) was used to investigate the effect of the excipient composition, binder amount and liquid to solid (L/S) ratio (independent variables) on drug dissolution rates, median particle size diameter and...
05. April 2017
Abstract A strategy based on sequential design of experiments (screening, optimization and confirmation) was used to develop a tablet formulation of ibuprofen (400 mg) that is manufactured by direct compression. This formulation has a high content of ibuprofen (76%), in spite of the poor flowability of the drug substance. Sequential design of experiments proved to be an effective and efficient strategy in formulation development.
07. February 2017
Abstract Niosomes entrapping pregabalin (PG) were prepared using span 60 and cholesterol in different molar ratios by hydration method, the remaining PG from the hydrating solution was separated from vesicles by freeze centrifugation. Optimization of nano-based carrier of pregabalin (PG) was achieved. Quality by Design strategy was successfully employed to obtain PG-loaded niosomes with the desired properties. The optimal particle size, drug release and entrapment efficiency were attained by...
20. December 2015
Amorphous nanoparticles are able to enhance the kinetic solubility and concomitant dissolution rates of BCS class II and BCS class II/IV molecules due to their characteristic increased supersaturation levels, smaller particle size and greater surface area. A DoE approach was applied to investigate formulation and spray drying process parameters for the preparation of spray dried amorphous ABT-102 nanoparticles. More
19. December 2015
The concept of Quality by Design is of increasing importance in the pharmaceutical industry, especially in the development of biotechnologically produced active pharmaceutical ingredients (APIs). These proteins/peptide-like materials are usually more sensitive than small molecules to the environmental and process parameters. More