- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
02. July 2018
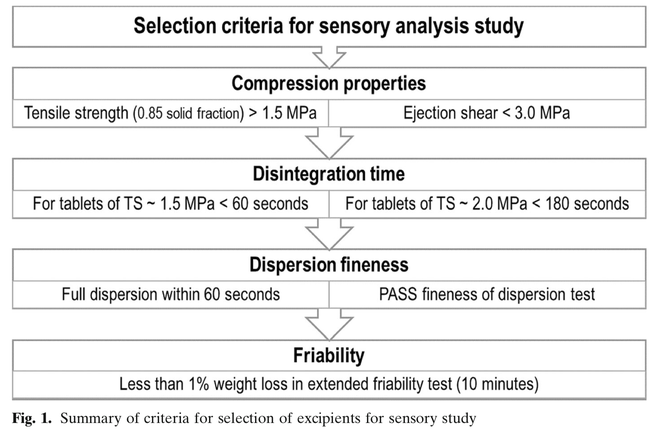
Palatability and patient acceptability are critical attributes of dispersible tablet formulation. Co-processed excipients could provide improved organoleptic profile due to rational choice of excipients and manufacturing techniques. The aim of this study was to identify the most suitable co-processed excipient to use within directly compressible dispersible tablet formulations. Nine excipients, selected based on successful manufacturability, were investigated in a randomised, preference and...
07. June 2018
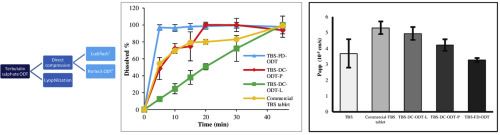
Asthma is a chronic respiratory condition characterized by attacks of spasm in the bronchi of the lungs, causing difficulty in breathing. Oral and inhalation routes are generally used for the treatment of asthma. Terbutaline sulfate (TBS), is a widely used bronchodilator for the treatment of asthma, is available in formulations in the market. However, there is no commercially available orally disintegrating tablets (ODTs) containing TBS. Therefore, this study was aimed to develop and...
09. December 2017
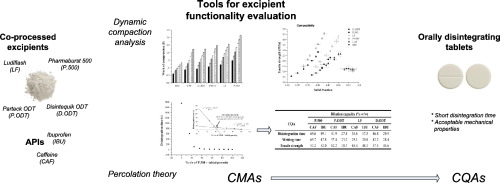
In the present work functional properties of new, co-processed excipients, Pharmaburst® 500, Parteck® ODT, Ludiflash® and Disintequik™ ODT, intended for direct compression of orally disintegrating tablets (ODTs), were investigated based on dynamic compaction analysis and percolation theory.
31. October 2017
Compaction of multiparticulates into tablets, particularly into orodispersible tablets (ODTs), is challenging. The compression of pellets, made by solid lipid extrusion/spheronization processes, presents peculiar difficulties since solid lipids usually soften or melt at relatively low temperature ranges and due to applied mechanical forces.
17. October 2017
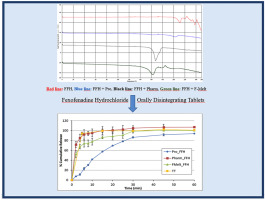
Allergic rhinitis is a common disease in children which has considerable negative effects on the quality of life. Fexofenadine hydrochloride (FFH) is a second-generation oral antihistamine which has been widely perscribed for alleviating symptoms of AR in children.
11. July 2017
Orally disintegrating tablets (ODT), are an innovative solid oral dosage form that is becoming more and more important in the pharmaceutical market, both for prescription and over-the-counter medications. In contrast to classical tablets, ODTs are specifically designed to disintegrate directly in the patient's mouth. The disintegrated tablet can then be swallowed easily without any resistance. BASF offers a comprehensive set of excipients that help you to easily formulate your active ingredient
12. May 2017
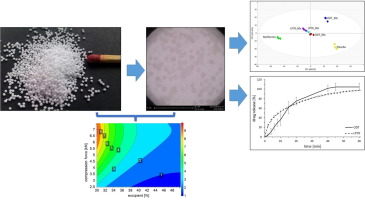
Pediatric, ibuprofen containing orodispersible tablets (ODTs) were prepared using the SeDeM expert system methodology. In order to facilitate formulation, directly compressible ibu- profen was employed (Ibuprofen DC 85TM) and character- ized using its SeDeM pro le. The mannitol based superdis- integrant Ludi ash® was characterized by the SeDeM-ODT expert system, which also allowed calculation of the opti- mal excipient concentration in order to obtain suitable tablet hardness and disintegration