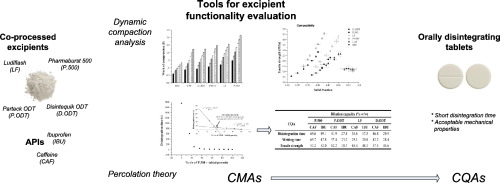
In the present work functional properties of new, co-processed excipients, Pharmaburst® 500, Parteck® ODT, Ludiflash® and Disintequik™ ODT, intended for direct compression of orally disintegrating tablets (ODTs), were investigated based on dynamic compaction analysis and percolation theory. Tablet disintegration time and mechanical properties have been recognized as critical quality attributes (CQAs) which should be optimized through pharmaceutical development. According to the obtained results, in order to achieve adequate mechanical resistance, excipients exhibiting high compactibility and tabletability are required, while, on the contrary, high porosity excipients with higher extent of elastic deformations, average tabletability and compactibility are necessary to obtain fast tablet disintegration. The results obtained in this study indicate that the most important excipient properties affecting tablet CQAs are porosity, mechanism of consolidation, compactibility and tabletability. Pharmaburst® 500, excipient with the highest elastic recovery, the lowest relative density, average compactibility and tabletability, and remarkably high dilution capacity (69.6% w/w of caffeine or 49.1% w/w of ibuprofen) exhibited favourable performance for ODT direct compression. Dynamic compaction analysis and percolation theory proved to be useful tools which can contribute to identification of the most important excipient functional properties influencing critical quality attributes of the prepared ODTs.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact