- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
21. July 2018
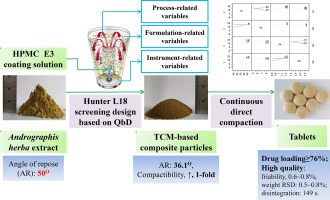
The Andrographis herba extract (AHE), a traditional Chinese medicine (TCM), was developed to directly compactible powders by fluid bed coating with 6% to 12% hydroxypropyl methylcellulose (HPMC). The process-, instrument-, and formulation-related variables of the coating process were simultaneously optimized with the Hunter L18 screening design. Yield (Y1), compactibility (Y2), and angle of repose (Y3) were measured as the responses. The optimized variables were 50 °C for inlet air...
11. April 2018
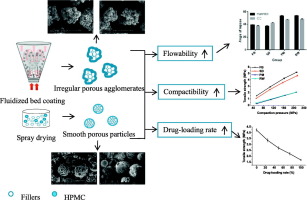
This study aimed to develop novel co-processed tablet fillers based on the principle of particle engineering for direct compaction and to compare the characteristics of co-processed products obtained by fluid-bed coating and co-spray drying, respectively. Water-soluble mannitol and water-insoluble calcium carbonate were selected as representative fillers for this study. Hydroxypropyl methylcellulose (HPMC), serving as a surface property modifier, was distributed on the surface of primary filler...
12. February 2018
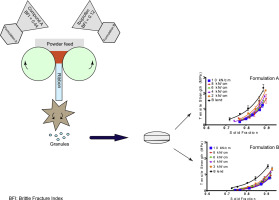
There is considerable interest in formulations with high active pharmaceutical ingredient (API) load, for reasons including lower patient tablet burden and therefore, potentially improved patient adherence. This remains a challenge not least because most APIs are poor flowing.
09. December 2017
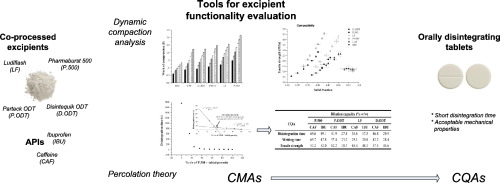
In the present work functional properties of new, co-processed excipients, Pharmaburst® 500, Parteck® ODT, Ludiflash® and Disintequik™ ODT, intended for direct compression of orally disintegrating tablets (ODTs), were investigated based on dynamic compaction analysis and percolation theory.
22. November 2016
Abstract The aim of this study was to prepare spherical agglomerates of lactose and to evaluate their physicochemical properties, flow properties, particle friability and compaction properties, and to compare them to commercially available types of lactose for direct compression (spray-dried, granulated and anhydrous β-lactose). Porous spherical agglomerates of α-lactose monohydrate with radially arranged prism-like primary particles were prepared exhibiting a high specific surface area. All...
11. October 2016
ABSTRACT Chitosan and Carbopol have been used to form a complex through an electrostatic interaction between the protonated amine (NH3+) group of chitosan and the carboxylate (COO-) group of Carbopol. In situ polyelectrolyte complexes formation based on the physical mixture of chitosan and sodium alginate were found and could be used as an oral controlled release matrix. The aim of this work is the assessment of a possible interaction between the particles of chitosan and Carbopol 974P NF that...
04. October 2016
The aim of this work is the assessment of the eventual enhancing effects of Carbopol 971P NF on the performance of Benecel K4M as a controlled release agent and its impact on other technological properties such as compactibility and powder flowability. The effect of Carbopol 971P NF and Benecel K4M in the performance of metronidazole tablets with controlled release was assessed using dissolution and compactibility profiles and the flowability of powders. Benecel K4M produces release profiles...