Abstract
There is considerable interest in formulations with high active pharmaceutical ingredient (API) load, for reasons including lower patient tablet burden and therefore, potentially improved patient adherence. This remains a challenge not least because most APIs are poor flowing.
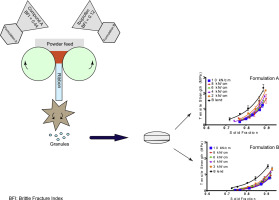
Roller compaction (RC) improves the flow of powders and is especially useful for APIs which are heat or moisture sensitive. However, due to loss of reworkability, RC results in reduction of tablet radial tensile strength (TS). To achieve a balance between flow and TS an understanding of material property and RC process parameters is needed. Material properties such as Brittle Fracture Index (BFI) govern a powder's behaviour during compression.
In this study; two APIs, ibuprofen (BFI = 0.12) and compound A (BFI = 0.44) were formulated at high load in an attempt to link BFI with the RC process. For both APIs, a roll force of 6 kN/cm was sufficient to attain granules of appropriate size and low fines. Ibuprofen formulation showed no conclusive correlation between roll force and flow, however, improved flow was seen in compound A formulation with increasing roll force. Both plastic-behaving ibuprofen and brittle compound A formulations demonstrated a reduction in tablet TS with increasing roll force. However, the ibuprofen formulation showed greater loss in reworkability.
Conclusion
This study aimed to understand the RC behavior of APIs with different brittleness classification; which in turn could inform key compaction targets such as ribbon solid fraction when formulating at high API load. Formulations containing brittle compound A (BFI = 0.44) pro- duced ribbons of higher tensile strength at a given solid fraction, in com- parison to formulations containing plastic ibuprofen (BFI = 0.12). Milled ribbons produced granules of larger particle size for both formu- lations, than the primary powder. A roll force 6 kN/cm attained granules of appropriate size and low fines and additional increase in roll force had no further tangible effect on particle size distribution. With regards to the flow of the granules, Formulation A appeared to improve in flow with increasing roll force however, the response of Formulation B to in- creasing roll force was inconclusive but remained as ‘easy flowing’. Both formulations showed loss of reworkability seen as a decrease in tablet radial tensile strength which correlated with increasing roll force used in RC. The brittle Formulation A, however, was less susceptible to reworkability loss due to its ability to form inter-particulate bridges with new surfaces formed during fragmentation. The formulation con- taining the plastic ibuprofen compressed relatively easily, achieving the target solid fraction under much lower compression stress. Al- though, the RC process did not have any effect on the compressibility of either formulation. The results demonstrate that while RC is a potentially applicable strategy in the production of tablets from formulations containing a high load of either a brittle or plastic API, consideration should be given to the targets of the ribbon solid fraction where a lower solid fraction target could be required for the plastic formulation to minimise the amount of deformation thereby allowing for a higher tablet radial tensile strength to be achieved.