- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
20. September 2018
The comparison of spray drying versus hot melt extrusion (HME) in order to formulate amorphous solid dispersions has been widely studied. However, to the best of our knowledge, the use of both techniques to form cocrystals within a carrier excipient has not previously been compared. The combination of ibuprofen (IBU) and isonicotinamide (INA) in a 1:1 molar ratio was used as a model cocrystal. A range of pharmaceutical excipients was selected for processing - mannitol, xylitol, Soluplus and PVP...
30. August 2018
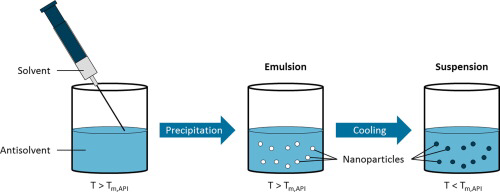
Antisolvent precipitation of poorly water-soluble drugs is a promising formulation technique to synthesize amorphous nanoparticles. The dissolution behavior of these nanoparticles is improved because of the high specific surface area and the amorphous state, leading to an enhanced bioavailability of the drug molecules. Nevertheless, stabilization of precipitated drug nanoparticles against agglomeration and recrystallization, which constitutes a key issue for further processing steps, has turned...
08. May 2018
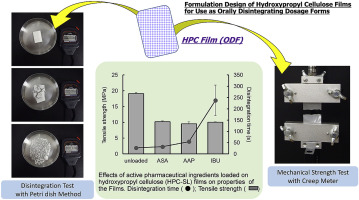
Hydroxypropyl cellulose (HPC) is a water-soluble polymer used as a binder during pharmaceutical tableting and granulation. HPC is also known as a base material for pharmaceutical film by virtue of its film formability with excellent plasticity. The aim of this study was to assess the applicability of HPC to orally disintegrating film (ODF) and to investigate optimization of the ODF formulation of HPC. The effects of the molecular weight of HPC and the addition of active pharmaceutical...
12. April 2018
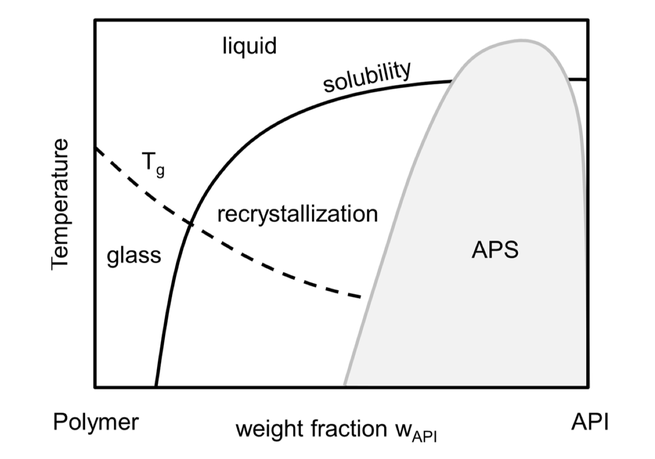
The long-term stability of pharmaceutical formulations of poorly-soluble drugs in polymers determines their bioavailability and therapeutic applicability. However, these formulations do not only often tend to crystallize during storage, but also tend to undergo unwanted amorphous-amorphous phase separations (APS). Whereas the crystallization behavior of APIs in polymers has been measured and modeled during the last years, the APS phenomenon is still poorly understood. In this study, the...
12. February 2018
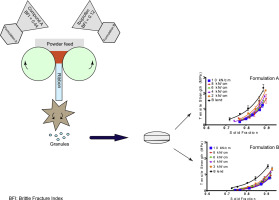
There is considerable interest in formulations with high active pharmaceutical ingredient (API) load, for reasons including lower patient tablet burden and therefore, potentially improved patient adherence. This remains a challenge not least because most APIs are poor flowing.
11. January 2018
The object of this study is to prepare and evaluate tablets with predesigned internal scaffold structure using 3D printing to achieve sustained drug release. Model drug (ibuprofen) and sustained release material (ethyl cellulose), together with other excipients, were firstly mixed and extruded into filaments by hot melt extrusion. Then these obtained filaments were printed into tablets by fused deposition modeling.
20. December 2017
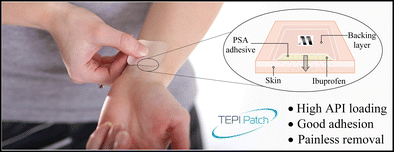
The main objective of this present study was the investigation of potential novel transdermal patch technology (TEPI®) delivering ibuprofen as the active pharmaceutical ingredient (API) using a novel poly(ether-urethane)-silicone crosslinked pressure-sensitive adhesive (PSA) as the drug reservoir in a solvent-free manufacturing process.
23. October 2017
Consumption of alcohol concomitantly with a drug may increase absorption of the active ingredients, leading to dose dumping. In this study, ibuprofen was administered to mice along with rice wine or beer. Blood concentrations of ibuprofen were lower when taken with alcohol than when taken with water.
10. October 2017
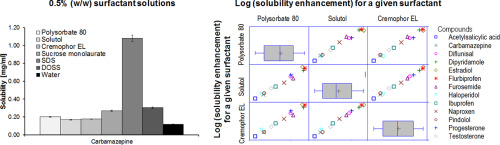
Solubility screening in different surfactant solutions is an important part of pharmaceutical profiling. A particular interest is in low surfactant concentrations that mimic the dilution of an oral dosage form.
29. September 2017
Ibuprofen is a non-steroidal anti-inflam-matory drug frequently administered to children of var-ious ages for relief of fever and pain and is approved as over-the-counter medication in many countries world-wide. Although there is extensive data on its efficacy and safety in children and adults, there are divergent dosing recommendations for analgesia and treatment of fever in infants, especially in the age group between 3 and 6 months of age.