- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
13. August 2018
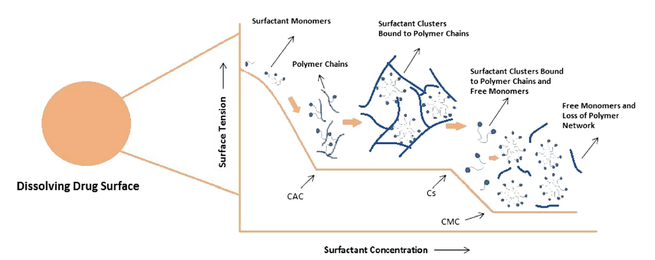
Surfactants are commonly incorporated in conventional and enabled formulations to enhance the rate and extent of dissolution of drugs exhibiting poor aqueous solubility. Generally the interactions between the drug and excipients are systematically evaluated, however, limited attention is paid towards understanding the effect of interaction between functional excipients and its impact on the performance of the product. In the current study, the effect of potential interaction between a nonionic...
17. March 2018
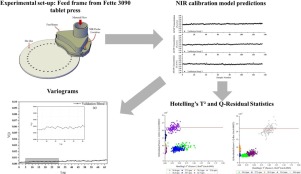
Near infrared (NIR) spectroscopy was used to determine the drug concentration in 3% (w/w) acetaminophen blends within the complex flow regime of the tablet press feed frame just before tablet compaction. NIR spectra also provided valuable information on the powder flow behavior within the feed frame and were used to track when a process enters or leaves the steady state.
19. February 2018
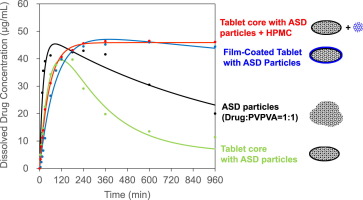
The effects of tablet preparation and subsequent film coating with amorphous solid dispersion (ASD) particles that were composed of a drug with poor water solubility and hydrophilic polymers were investigated. ASD particles were prepared with a drug and vinylpyrrolidone–vinyl acetate copolymer (PVPVA) or polyvinylpyrrolidone (PVP) at a weight ratio of 1:1 or 1:2 using a melt extrusion technique. Tablets were prepared by conventional direct compression followed by pan coating. A mathematical...
12. February 2018
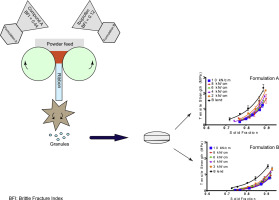
There is considerable interest in formulations with high active pharmaceutical ingredient (API) load, for reasons including lower patient tablet burden and therefore, potentially improved patient adherence. This remains a challenge not least because most APIs are poor flowing.
05. February 2018
Children requiring cortisol replacement therapy are often prescribed hydrocortisone doses of
2.5 mg, but as this is commercially unavailable 10 mg tablets, with functional break lines, are split commonly in an attempt to deliver the correct dose. This study aimed
to determine the dose variation obtained from quartered hydrocortisone tablets when different operators performed the splitting procedure and to ascertain whether better uniformity could be attained from mini-tablets as an alternative f
17. January 2018
Controlled release dosage forms are essential in certain dosage regimens where release rate of drug impacts the effectiveness for therapy. In the present study, modified release dosage form of Domperidone was developed in the form of pellets which were subsequently compressed into tablet to disintegrate and swallow by patients without changing the release profile.
03. January 2018
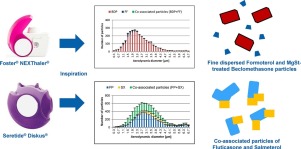
The in vitro aerosol performance of two combination dry powder inhaler (DPI) products, Foster® NEXThaler® and Seretide® Diskus® were investigated with single particle aerosol mass spectrometry (SPAMS). The in-vitro pharmaceutical performance is markedly different for both inhalers. Foster® NEXThaler® generates a higher fine particle fraction (FPF <5 μm) and a much higher relative extra fine particle fraction (eFPF <2 μm).
17. October 2017
ARMOR PHARMA™ lactose monohydrate 350M is a fine milled powder of α-lactose monohydrate.
Higly compressible, this grade is mostly intended to be used in tablets formulation by Wet or Dry granulation.
11. September 2017
ARMOR PHARMA - technological lactose excipients - currently markets 3 types of pharmaceutical lactose.
ARMOR PHARMA™ lactose monohydrate : sieved & milled lactose
EXCIPRESS™ lactose for Direct Compression
EXCIPURE™ lactose for Dry powder Inhalation