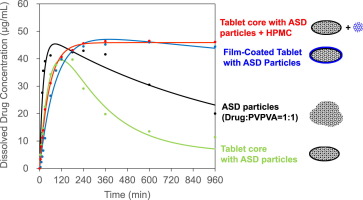
The effects of tablet preparation and subsequent film coating with amorphous solid dispersion (ASD) particles that were composed of a drug with poor water solubility and hydrophilic polymers were investigated. ASD particles were prepared with a drug and vinylpyrrolidone–vinyl acetate copolymer (PVPVA) or polyvinylpyrrolidone (PVP) at a weight ratio of 1:1 or 1:2 using a melt extrusion technique. Tablets were prepared by conventional direct compression followed by pan coating. A mathematical model based on the Noyes–Whitney equation assuming that stable crystals precipitated at the changeable surface area of the solid–liquid interface used to estimate drug dissolution kinetics in a non-sink dissolution condition. All the ASD particles showed a maximum dissolution concentration approximately ten times higher than that of the crystalline drug. The ASD particles with PVPVA showed higher precipitation rate with lower polymer ratio, while PVP did not precipitate within 960 min regardless of the polymer ratio, suggesting the ASD particles of 1:1 drug:PVPVA (ASD-1) were the most unstable among the ASD particles considered. The dissolution of a core tablet with ASD-1 showed less supersaturation and a much higher precipitation rate than those of ASD-1 particles. However, a film-coated tablet or core tablet with a trace amount of hydroxypropylmethylcellulose (HPMC) showed a similar dissolution profile to that of the ASD-1 particles, indicating HPMC had a remarkable precipitation inhibition effect. Overall, these results suggest that tablet preparation with ASD may adversely affect the maintenance of supersaturation; however, this effect can be mitigated by adding an appropriate precipitation inhibitor to the formulation.
Conclusions
The effect of tablet formation with ASD particles on the supersaturation dissolution profiles of the drug FK555 was evaluated. Hiram’s mathematical model was a practical tool to differentiate the dissolution profiles of these formulations with high Gibb’s free energies, including actual dosage forms (i.e., tablets). In particular, this model did not require predetermination of physical parameters of the drug and dosage form. The tablet preparation without a film coating negatively affected drug dissolution properties, mainly because of the enhancement of the drug precipitation kinetics during tablet dissolution. However, we found that drug precipitation can be inhibited by applying a film coating onto the tablet core. This study also revealed that precipitation was inhibited by the addition of a trace amount of HPMC.