- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
09. December 2017
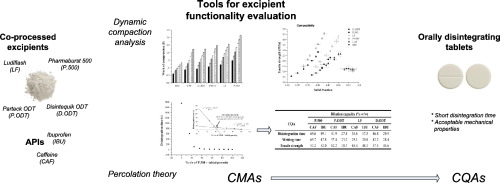
In the present work functional properties of new, co-processed excipients, Pharmaburst® 500, Parteck® ODT, Ludiflash® and Disintequik™ ODT, intended for direct compression of orally disintegrating tablets (ODTs), were investigated based on dynamic compaction analysis and percolation theory.
03. February 2017
ABSTRACT Co-processing is one of the ways to develop new excipients for the pharmaceutical industry. Co-processed excipients are a combination of established two or more excipients through physical mixing or co-process technology. Co-processed excipients has no change in chemical structure, it only changes physical properties of the final product. Co-processing technology could lead to formation of co-processed excipient with superior physical properties compared to simple physical mixtures of...
08. January 2017
ABSTRACT: In the present study, novel co-processed superdisintegrants were developed by spray drying method using microcrystalline cellulose and mannitol in different ratios (1:1, 1:2 and 1:3) for use in the fast dissolving tablet formulations. The developed excipients were evaluated for angle of repose, Carr’s index and Hausner’s ratio in comparison with physical mixture of superdisintegrants. The angle of repose of the developed excipients was found to be < 30o, Carr’s index in the...
19. October 2016
Interessieren Sie sich für alternative Formulierungsansätze bei der Direkttablettierung? Dann sollten Sie auf gar keinen Fall den Vortrag von Albrecht Krämer von Meggle verpassen. Auf dem Pharmafeststoff-Forum hält er einen Vortrag über die Qualitätsvorteile dieses Formulierungsansatzes. Nach einem kurzen Überblick über die historische Entwicklung der "Co-processed Excipients", deren generelle Herstellungsmethoden sowie deren Entwicklung folgt eine Übersicht über typische...
09. August 2016
Abstract The aim of this study is to present the possibility of using of co-processed dry binders for formulation of matrix tablets with drug controlled release. Hydrophilic matrix tablets with tramadol hydrochloride, hypromellose and different co-processed dry binders were prepared by direct compression method. Hypromelloses Methocel™ K4M Premium CR or Methocel™ K100M Premium CR were used as controlled release agents and Prosolv® SMCC 90 or Disintequik™ MCC 25 were used as co-processed...
08. August 2016
The present investigation was carried out to develop and characterize a multifunctional co-processed excipient for improving the compressibility of poorly compressible drugs. Etodolac was used as a model drug. Microcrystalline cellulose (MCC), lactose monohydrate (lactose), and StarCap 1500 (StarCap) were selected as components of the co-processed excipient. The spray drying method was used for co-processing of excipients. D-optimal mixture design was applied to optimize the proportion of...
04. June 2016
The global excipients market should reach nearly $6.9 billion by 2020 from approximately $6.3 billion in 2015, according to a new report from BCC Research, LLC. Growth in the demand for pharmaceuticals and biopharmaceuticals should drive market growth in the excipients market as will the development of innovative drugs for chronic diseases and an increase in generic drug production. Increased research and development spending, growing competition, looming patent expiries, new technologies, and...
09. April 2016
Sumatriptan succinate (SS) is a selective serotonin receptor agonist used for the treatment of migraine attacks, suffering from extensive first-pass metabolism and low oral bioavailability (∼14%). The aim of this work is to compare the performance of different ready-made co-processed platforms (Pharmaburst®, Prosolv ODT®, Starlac®, Pearlitol Flash®, or Ludiflash®) in the formulation of SS sublingual orodispersible tablets (ODTs) using direct compression technique. The prepared SS ODT...
16. February 2016
This study investigates the extrusion-spheronization performance of some mixtures of co-processed κ-carrageenan and pectin (as excipient), and sodium starch glycolate (as superdisintegrant). Attention is focused with an objective to improve the mechanical stability and the dissolution rate of poorly-soluble domperidone (as a model drug). Initially co-processed κ-carrageenan- pectin excipient is prepared with different ratios of κ-carrageenan and pectin. Different marketed brands of...
07. November 2015
FELICITAS GUTH*, HEIKO A. SCHIFFTER, KARL KOLTER *Corresponding author BASF SE, Formulation Nutrition & Health, Carl Bosch Str. 38, 67056 Ludwigshafen, Germany More