- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
15. May 2018
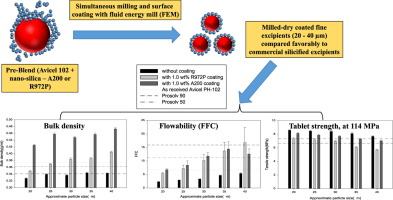
Asolventless process for simultaneously milling and dry coating microcrystalline cellulose (MCC) was investigated for producing fine excipients in five different sizes (∼20, 25, 30, 35, 40µm) havinghigh bulk density (BD), good flow function coefficient (FFC), and excellent compaction. Avicel PH-102, used as the starting material, was milled and coated with two grades of silicas, hydrophobic andhydrophilic (R972P and A200), using a fluid energy mill (FEM). Through judicious selection of the...
02. February 2018
The purpose of the research described herein was to develop a kinetic model for quantifying the effects of conditional and compositional variations on non-covalent polymorphic and covalent chemical transformations of gabapentin.
24. December 2017
Preprocessing of pharmaceutical powders is a common procedure to condition the materials for a better manufacturing performance. However, such operations may induce undesired material properties modifications when conditioning particle size through milling, for example. Modification of both surface and bulk material structure will change the material properties, thus affecting the processability of the powder.
05. September 2017
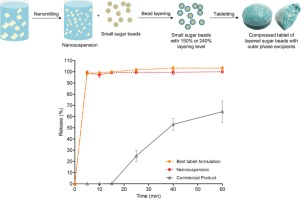
Abstract There has been limited research done on the downstream processing of nanosuspensions into solid oral dosage forms. This paper demonstrates the bead layering process with a layering level at 150% and 240%, as well as the selection and justification of the outer phase excipients for tabletability and disintegrating properties. In a previous study, an itraconazole nanosuspension stabilised by SDS and HPMC E5 was layered onto sugar beads with coating polymer HPMC VLV. In the current study,...
31. May 2017
Praziquantel, a BCS II class anthelmintic drug used for the treatment of schistosome infections, was coground in a vibrational mill with different polymers (linear and crosslinked povidone, copovidone and sodium starch glycolate
18. April 2017
Abstract There is more research required to broaden the knowledge on the downstream processing of nanosuspensions into solid oral dosage forms, especially for coated nanosuspensions onto beads as carriers. This study focuses on bead layering as one approach to solidify nanosuspensions. The aim was to systematically investigate the influence of type of coating polymer (HPMC VLV vs. copovidone), bead material and bead size (sugar vs. MCC, and small vs. large) and coating thickness (50%–150%...
06. April 2017
Abstract With increasing importance of continuous manufacturing, the interest in integrating dry granulation into a continuous manufacturing line is growing. Residence time distribution measurements are of importance as they provide information about duration of materials within the process. These data enable traceability and are highly beneficial for developing control strategies. A digital image analysis system was used to determine the residence time distribution of two materials with...
02. April 2017
Abstract Dissolution of bicalutamide processed with polyvinylpyrrolidone by either supercritical carbon dioxide or ball milling has been investigated. Various compositions as well as process parameters were used to obtain binary systems of the drug with the carrier. Thermal analysis and powder X-ray diffractometry confirmed amorphization of bicalutamide mechanically activated by ball milling and the decrease in crystallinity of the supercritical carbon dioxide-treated drug. Both methods led to...
06. March 2017
Abstract The present study investigates the effects of formulation and process parameters on the production of aprepitant nanosuspensions applying wet media milling and subsequent solidification. Six stabilizers were used: two brands of hydroxylpropylmethyl cellulose (HPMC E-15LV and Pharmacoat 603), hydroxypropyl cellulose (HPC-SSL), polyvinylpyrollidone (PVP), D-α-Tocopherol polyethylene glycol 1000 succinate (TPGS 1000), Poloxamer P188 and sodium dodecyl sulfate (SDS), while two diluents...
14. February 2017
Abstract Dry granulation using roll compaction is a typical unit operation for producing solid dosage forms in the pharmaceutical industry. Dry granulation is commonly used if the powder mixture is sensitive to heat and moisture and has poor flow properties. The output of roll compaction is compacted ribbons that exhibit different properties based on the adjusted process parameters. These ribbons are then milled into granules and finally compressed into tablets. The properties of the ribbons...