- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
26. July 2018
Paediatric specific medicines have become increasingly researched since the introduction of paediatric investigation plan requirements in 2007. Various dosage forms continue to be investigated for their appropriateness for children, including multiparticulates. Multiparticulates are currently available as tablets, capsules, sachets and medicated spoons/straws/syringes. These presentations offer limited dose flexibility with some only providing a singlefixed dose. A device capable of repeated...
31. December 2017
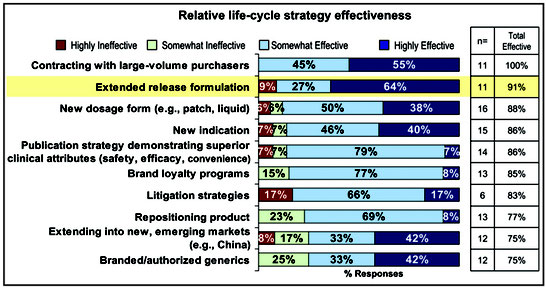
The FDA clarified the regulatory thicket surrounding the 505(b)(2) approval pathway for drug formulations just over a decade ago. In the intervening years, this pathway has become an increasingly popular way for drug makers to commercialize products. The 505(b)(2) pathway allows companies to make modest changes to an already approved drug and get continued market exclusivity for from three to as many as seven years.
22. November 2017
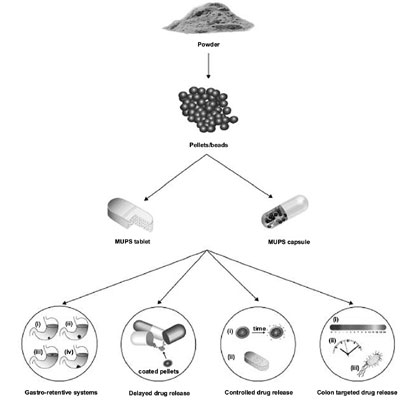
Background and Objective: Single-unit solid oral dosage forms such as tablets and capsules are considered the most common and acceptable form of immediate release systemic drug delivery systems. On the other hand, multiple-unit pellet systems (MUPS) have in recent years become an important dosage form that offers various advantages over conventional single-unit solid oral dosage forms.
21. November 2017
Process Analytical Technology, or “PAT” is the term given to analytical instruments developed to measure certain attributes of product within the manufacturing process, eliminating, or substantially minimising the need for sampling for off-line analysis. This approach has several key advantages over traditional off-line analysis methods and includes process measurements in situ with instant access to data which facilitates rapid decisions during product development and manufacture.
19. September 2017
Fluid bed coating offers potential advantages as a formulation platform for amorphous solid dispersions (ASDs) of poorly soluble drugs, being a one-step manufacturing process which could reduce therisk of phase separation associated with multiple step manufacturing approaches. However, the impact of the physicochemical nature of carrier spheres on the properties and drug release from the ASDshas not been studied in detail. In this work, tartaric acid (TAP) and microcrystalline cellulose (CEL)...
14. September 2017
Prof Martin Whitaker (The University of Sheffield) will present Infacort®: a PUMA success story at the 9th conference of the European Paediatric Formulation Initiative (EuPFI) in Warsaw (Poland) on Thursday 21st September 2017 at 12:25.
05. September 2017
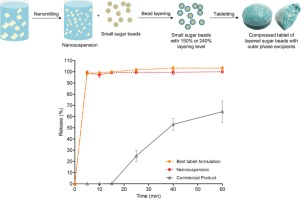
Abstract There has been limited research done on the downstream processing of nanosuspensions into solid oral dosage forms. This paper demonstrates the bead layering process with a layering level at 150% and 240%, as well as the selection and justification of the outer phase excipients for tabletability and disintegrating properties. In a previous study, an itraconazole nanosuspension stabilised by SDS and HPMC E5 was layered onto sugar beads with coating polymer HPMC VLV. In the current study,...
23. August 2017
The multiparticulate drug product concept covering micropellets, pellets, and mini-tablets is presented as a highly feasible approach to present convenient and patient friendly medication for the geriatric population. Improved swallowability and optimized administration regimen going along with defined drug dosage are achievable.
05. July 2017
Acid-labile drugs are easily degraded in acidic medium, which have been mainly formulated as enteric-coated dosage forms for oral administration. The objective of this study was to prepare oral multiparticulate formulations of acid-labile drugs, using ilaprazole, lansoprazole and rabeprazole sodium as model drugs.
29. June 2017
Process Analytical Technology, or "PAT" is the term given to analytical instruments developed to measure certain attributes of product within the manufacturing process, eliminating, or substantially minimising the need for sampling for off-line analysis