- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
15. August 2018
Pharmaceutical tablets contain a variety of active substances and excipients which could be monitored by Raman spectroscopy/microscopy. Raman microscopic spectra are affected not only by the sample composition but also by preparation/measurement conditions. The character and variability of surface morphology (of tablet slices), the appropriate levels of focusing and other adjustable settings of the measurement instrumentation represent important parameters to be considered for obtaining...
17. July 2018
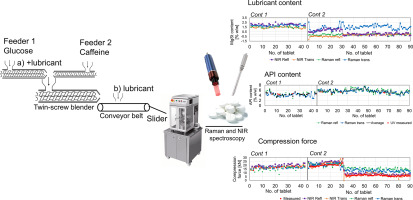
By the advent of continuous pharmaceutical manufacturing, fast and accurate characterization of product quality has become of a major interest. Although it also promotes the real-time release testing approach, so far mainly content uniformity studies were performed by near-infrared (NIR) spectroscopy. This paper proposes the simultaneous application of NIR and Raman spectroscopy to nondestructively analyze the critical quality attributes of continuously produced tablets in a real-time release...
15. October 2017
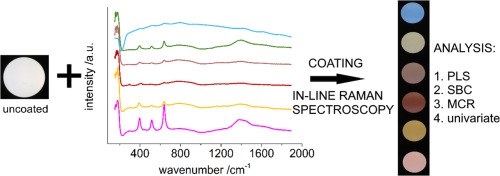
Endpoints of coating processes for colored tablets were determined using in-line Raman spectroscopy. Coatings were performed with six commercially available formulations of pink, yellow, red, beige, green and blue color. The coatings were comprising pigments and/or dyes, some causing fluorescence and interfering the Raman signal. Using non-contact optics, a Raman probe was used as process analytical technology (PAT) tool, and acquired spectra were correlated to the sprayed mass of aqueous...
14. October 2017
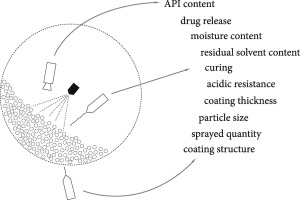
Over the last two decades, regulatory agencies have demanded better understanding of pharmaceutical products and processes by implementing new technological approaches, such as process analytical technology (PAT). Process analysers present a key PAT tool, which enables effective process monitoring, and thus improved process control of medicinal product manufacturing. Process analysers applicable in pharmaceutical coating unit operations are comprehensibly described in the present article. The...
14. June 2016
Abstract Investigation of downstream processing of nanofibrous amorphous solid dispersions to generate tablet formulation is in a quite early phase. Development of high speed electrospinning opened up the possibility to study tableting of electrospun solid dispersions (containing polyvinylpyrrolidone-vinyl acetate and itraconazole [ITR] in this case). This work was conducted to investigate the influence of excipients on dissolution properties and the feasibility of scaled-up rotary press...
09. April 2016
For effective topical delivery, a drug must cross the stratum corneum (SC) barrier into viable tissue. The use of permeation enhancers is a widespread approach for barrier modification. In the current study, flufenamic acid (FluA), a non-steroidal anti-inflammatory drug, is a model agent for investigating the influence of hydrophobic versus hydrophilic enhancers. In separate experiments, FluA in octanol or propylene glycol/ethanol (75/25) is applied to the SC for varying times followed by...
22. March 2016
Three microcrystalline cellulose (MCC) samples were manufactured from bleached and unbleached softwood kraft pulp, and their properties were compared to those of the commercial MCC, Avicel PH-101. One of the produced samples retained a large portion of lignin (10.3%), while the two others retained only some. The physical, chemical, thermogravimetric, and molecular properties were analyzed. The presence of lignin caused a substantial effect on the thermogravimetric and chemical properties of the...
18. February 2016
Purpose: Introduction to hot melt co-extrusion for utilization as a drug delivery method. Influence of process parameters on final drug product quality. Application of Raman imaging microscopy as a analytical tool for quality control of the co-extrudate. Methods: Co-extrusion with a newly designed co-extrusion die and a pharmaceutical relevant polymer/active formulation. Chemical mapping with Raman microscopy to provide evidence on extrudate integrity and absence of drug migration. Results: The...
07. February 2016
In the pharmaceutical industry, dextrose is used as an active ingredient in parenteral solutions and as an inactive ingredient (excipient) in tablets and capsules. In order to address the need for more sophisticated analytical techniques, we report our efforts to develop enhanced identification methods to screen pharmaceutical ingredients at risk for adulteration or substitution using field-deployable spectroscopic screening. In this paper we report our results for a study designed to evaluate...
24. September 2015
The article describes the application of a twin-screw granulation process to enhance the dissolution rate of the poorly water soluble drug, ibuprofen (IBU). A quality-by-design (QbD) approach was used to manufacture IBU loaded granules via hot-melt extrusion (HME) processing. More