- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
08. August 2018
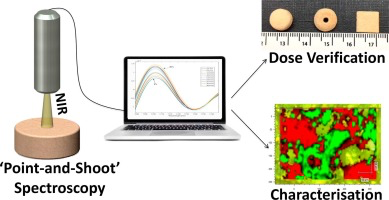
Three-dimensional printing (3DP) has the potential to cause a paradigm shift in the manufacture of pharmaceuticals, enabling personalised medicines to be produced on-demand. To facilitate integration into healthcare, non-destructive characterisation techniques are required to ensure final product quality. Here, the use of process analytical technologies (PAT), including near infrared spectroscopy(NIR) and Raman confocal microscopy, were evaluated on paracetamol-loaded 3D printed cylindrical...
17. July 2018
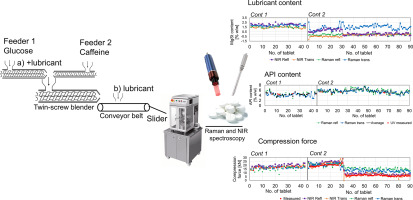
By the advent of continuous pharmaceutical manufacturing, fast and accurate characterization of product quality has become of a major interest. Although it also promotes the real-time release testing approach, so far mainly content uniformity studies were performed by near-infrared (NIR) spectroscopy. This paper proposes the simultaneous application of NIR and Raman spectroscopy to nondestructively analyze the critical quality attributes of continuously produced tablets in a real-time release...
25. April 2018
To support the practical implementation of the ICH Q3D guideline, which describes a risk-based approach to the control of elemental impurities in drug products, a consortium of pharmaceutical companies has established a database to collate the results of analytical studies of the levels of elemental impurities within pharmaceutical excipients. This database currently includes the results of 26723 elemental determinations for 201 excipients and represents the largest known, and still rapidly...
05. March 2018
This study presents a framework for process and product development on a continuous direct compression manufacturing platform. A challenging sustained release formulation with high content of a poorly flowing low density drug was selected. Two HPMC grades were evaluated as matrix former: standard Methocel CR and directly compressible Methocel DC2. The feeding behavior of each formulation component was investigated by deriving feed factor profiles.
01. March 2018
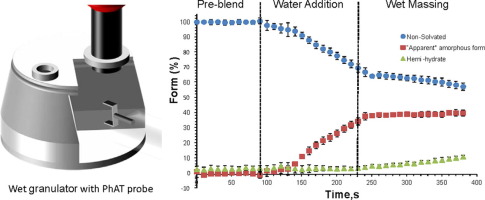
Form changes during drug product processing can be a risk to the final product quality in terms of chemical stability and bioavailability. In this study, online Raman spectroscopy was used to monitor the form changes in real time during high shear wet granulation of Compound A, a highly soluble drug present at a high drug load in an extended release formulation. The effect of water content, temperature, wet massing time and drying technique on the degree of drug transformation were examined.
07. November 2017
Over the last few decades, hot melt extrusion (HME) has emerged as a successful technology for a broad spectrum of applications in the pharmaceutical industry. As indicated by multiple publications and patents, HME is mainly used for the enhancement of solubility and bioavailability of poorly soluble drugs. This review is focused on the recent reports on the solubility enhancement via HME and provides an update for the manufacturing/scaling up aspects of melt extrusion. In addition, drug...
15. October 2017
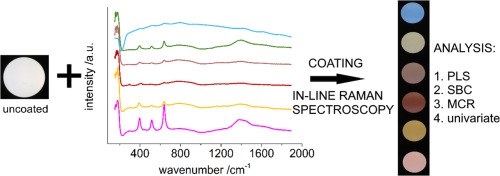
Endpoints of coating processes for colored tablets were determined using in-line Raman spectroscopy. Coatings were performed with six commercially available formulations of pink, yellow, red, beige, green and blue color. The coatings were comprising pigments and/or dyes, some causing fluorescence and interfering the Raman signal. Using non-contact optics, a Raman probe was used as process analytical technology (PAT) tool, and acquired spectra were correlated to the sprayed mass of aqueous...
14. October 2017
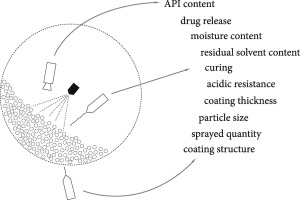
Over the last two decades, regulatory agencies have demanded better understanding of pharmaceutical products and processes by implementing new technological approaches, such as process analytical technology (PAT). Process analysers present a key PAT tool, which enables effective process monitoring, and thus improved process control of medicinal product manufacturing. Process analysers applicable in pharmaceutical coating unit operations are comprehensibly described in the present article. The...
13. April 2017
Abstract Microwave resonance technology (MRT) is known as a process analytical technology (PAT) tool for moisture measurements in fluid-bed granulation. It offers a great potential for wet granulation processes even where the suitability of near-infrared (NIR) spectroscopy is limited, e.g. colored granules, large variations in bulk density. However, previous sensor systems operating around a single resonance frequency showed limitations above approx. 7.5% granule moisture. This paper describes...
29. March 2017
ABSTRACT: Control of elemental impurities in pharmaceutical materials is currently undergoing a transition from control based on concentrations in components of drug products to control based on permitted daily exposures in drug products.Within the pharmaceutical community, there is uncertainty regarding the impact of these changes on manufactures of drug products. This uncertainty is fueled in part by a lack of publically available information on elemental impurity levels in common...