- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
14. October 2017
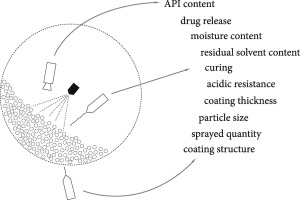
Over the last two decades, regulatory agencies have demanded better understanding of pharmaceutical products and processes by implementing new technological approaches, such as process analytical technology (PAT). Process analysers present a key PAT tool, which enables effective process monitoring, and thus improved process control of medicinal product manufacturing. Process analysers applicable in pharmaceutical coating unit operations are comprehensibly described in the present article. The...
20. September 2017
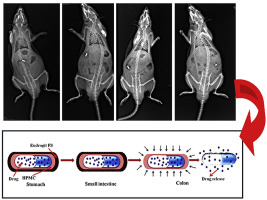
Abstract The objective of this study was to evaluate the combination of pH-dependent and time-dependent polymers on drug release in order to optimize coating for colonic delivery. Response surface methodology (RSM) based on central composite design (CCD) was employed for formulation optimization. Theophylline was used as model drug and Eudragit® FS 30D and hydroxypropyl methylcellulose (HPMC) were used as pH-dependent and time-dependent polymer, respectively. Dissolution test was carried out...
12. March 2017
Abstract Novel excipients are entering the market to enhance the bioavailability of drug particles by having a high porosity and thus providing a rapid liquid uptake and disintegration to accelerate subsequent drug dissolution. One example of such a novel excipient is functionalised calcium carbonate (FCC), which enables the manufacture of compacts with a bimodal pore size distribution consisting of larger inter-particle and fine intra-particle pores. Five sets of FCC tablets with a target...
04. October 2016
Abstract In the present study the applicability of multispectral UV imaging in combination with multivariate image analysis for surface evaluation of MUPS tablets was investigated with respect to the differentiation of the API pellets from the excipients matrix, estimation of the drug content as well as pellet distribution, and influence of the coating material and tablet thickness on the predictive model. Different formulations consisting of coated drug pellets with two coating polymers...
21. September 2016
Purpose Investigate the extended release behaviour of compacts containing mixtures of hydrophilic HPMC and PEO in hydrating media of differing ionic strengths. Methods The extended release behaviour of various HPMC:PEO compacts was investigated using dissolution testing, confocal microscopy and magnetic resonance imaging, with respect to polymer ratio and ionic strength of the hydrating media. Results Increasing HPMC content gave longer extended release times, but a greater sensitivity to high...
24. March 2016
The increasing demand for personalized medicine necessitates the production of easily customizable dosage forms. As the number of possible dosage forms may scale towards infinity, their uniqueness require a versatile production platform and numerical simulation in order to be manufactured efficiently. A mathematical description of these systems is the only feasible approach to manage such diverse properties of different products. However, experimental verification is still essential for...
18. February 2016
Purpose: Introduction to hot melt co-extrusion for utilization as a drug delivery method. Influence of process parameters on final drug product quality. Application of Raman imaging microscopy as a analytical tool for quality control of the co-extrudate. Methods: Co-extrusion with a newly designed co-extrusion die and a pharmaceutical relevant polymer/active formulation. Chemical mapping with Raman microscopy to provide evidence on extrudate integrity and absence of drug migration. Results: The...
16. February 2016
Disintegrant is one of the most important components in a typical tablet dosage form. It is responsible for ensuring the break-up of the tablet matrix upon ingestion. Disintegrants act by different mechanisms, and a number of factors may affect their performance. It is important for formulators to understand how disintegrants function so as to be able to judiciously use disintegrants to develop optimized formulations. If the formulator is required to implement the quality by design paradigm...
25. January 2016
Recently, we reported that intranasal vaccination of humans with whole inactivated influenza vaccine in the absence of mucosal adjuvant induced neutralizing antibody responses in the serum and nasal mucus. The mucoadhesive excipient carboxy-vinyl polymer (CVP) increases the viscosity and therefore mucoadhesiveness of intranasal medicaments and is an authorized excipient in Japan. In the present study, we analyzed the effect of adding CVP on intranasal whole inactivated influenza vaccine antigen...
11. January 2016