- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
28. May 2018
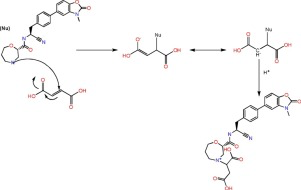
During compatibility study of the AZD7986 project, a peak of 3 area% at the tail (RRT 1.03) of the active pharmaceutical ingredient (API) was discovered for all tablets containing sodium stearyl fumarate (PRUV) under humid condition (e.g. 50 °C/75% RH), regardless of choice of disintegrant or filler combination. The degradant was needed to be identified to understand the corresponding reaction mechanism and help the final formulation design. Structure elucidation was therefore done by...
03. May 2018
The U.S. Pharmacopeia defines excipients as substances other than the active pharmaceutic ingredient (API) that are added in a drug delivery system in order to aid in the manufacturing process and enhance stability, bioavailability, safety, effectiveness and delivery of the drug. The 1968 phenytoin intoxication outbreak in Brisbane, Australia, is a classic example of an API-excipient interaction. When administered with CaSO4 the absorption of phenytoin was reduced due to an interaction between...
16. September 2017
The choice of excipients constitutes a major part of preformulation and formulation studies during the preparation of pharmaceutical dosage forms. The physical, mechanical, and chemical propertiesofexcipients affect various formulation parameters, such as disintegration, dissolution, and shelf life, and significantly influence the final product. Therefore, several studies have been performedtoevaluate the effect of drug-excipient interactions on the overall formulation. This article reviews the...
06. June 2016
Application of thermogravimetry (TG) alone to study compatibility/incompatibility of active pharmaceutical ingredients (APIs) with excipients yields to misleading results due to overlapping of the thermal stages in the course of decomposition of both ingredients and their pharmaceutical mixtures. Hence, the purpose of this study was to assess the usefulness of multivariate statistical analysis as a supporting tool for interpretation of the TG traces during assessing compatibility of...
09. March 2016
Abstract: Lubrication plays a key role in successful manufacturing of pharmaceutical solid dosage forms; lubricants are essential ingredients in robust formulations to achieve this. Although many failures in pharmaceutical manufacturing operations are caused by issues related to lubrication, in general, lubricants do not gain adequate attention in the development of pharmaceutical formulations. In this paper, the fundamental background on lubrication is introduced, in which the relationships...
24. December 2015
06. August 2015
Drug excipient physicochemical characterization is a systematic approach towards design of therapeutically active and stable dosage forms. The rapid advancements in novel drug delivery systems have led to an interest by formulation scientists in the role and functionality of the excipients. Fourier transform infrared spectroscopy (FTIR), Differential scanning calorimetry (DSC), and Powder X-ray diffractometry (XRD) analytical techniques of high resolution were used to get an insight on solid...