- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
30. June 2016
Abstract: In HIV-1 management, eradication of the virus from sanctuaries represents a major and challenging goal. The genital tract, gut associated lymphoid tissue, lymph nodes, central nervous system, macrophages and latently infected CD4+ T lymphocytes are typical sites where HIV-1 compartmentalizes. To circumvent this problem, a consistent number of studies have focused on improving ARVs (antiretroviral drugs) delivery into sanctuary sites and different nanotechnological approaches have been...
30. June 2016
Abstract Age-related pharmacological changes complicate oral dosage form (ODF) suitability for older adults. The aim of this study was to investigate the appropriateness of ODF for older adults by determining the prevalence of ODF modifications in an aged care facility in Ireland. Drug charts for eligible patients were obtained. Details of all medications administered were recorded. ODF modifications were examined to determine if they were evidence-based: defined as complying with the product...
30. June 2016
Abstract In the development of transdermal and topical products it is important to understand how formulation ingredients interact with the molecular components of the upper layer of the skin, the stratum corneum (SC), and thereby influence its macroscopic barrier properties. The aim here was to investigate the effect of two commonly used excipients, transcutol and dexpanthenol, on the molecular as well as the macroscopic properties of the skin membrane. Polarization transfer solid-state NMR...
27. June 2016
Abstract Pharmaceutical excipients contribute unique functionalities to formulations, thereby largely determining the drug products quality and influencing its safety and efficacy. Changes and variations of excipients in licensed products are, therefore, placed under strict regulatory control. This article presents a proposed regulatory mechanism for pharmaceutical excipients by United States of Food and Drug Administration (USFDA). In Unites States, excipients are regulated by United States of...
27. June 2016
In the context of the revision of the guideline on ‘Excipients in the label and package leaflet of medicinal products for human use’ (CPMP/463/00 Rev. 1) Draft report published in support to the Q&A document. For information only
27. June 2016
In the context of the revision of the guideline on ‘Excipients in the label and package leaflet of medicinal products for human use’ (CPMP/463/00 Rev. 1) Draft report published in support to the Q&A document. For information only
27. June 2016
Abstract A medicine consists of 2 fundamental parts: the active pharmaceutical ingredient and the excipient. Most, if not all, medicines could not be made without the use of excipients. In the early times, the safety of excipients was overlooked and no specific safety tests were generally conducted. This fact has been changed over times and is currently being recognized that the excipient's toxicity is not negligible, because its direct interaction with the active pharmaceutical ingredient or...
25. June 2016
A Solvent-Free Advanced Productivity Technology for Barrier Membrane Coatings Philadelphia, PA: Tuesday, June 21, 2016 “The global commercial launch of ETHOCEL™ HP is an exciting development for our customers, who continue to demand more efficient, sustainable solutions to help improve their products and their manufacturing,” said Marc Van Gerwen, business director for Dow Pharma & Food Solutions. “From idea to market – all in less than two years – ETHOCEL™ HP is another...
25. June 2016
Abstract Solvent-free hot-melt coating processing is a novel and cost-efficient approach to manufacturing taste-masked multiparticulate systems. However, most API powders are fine and cohesive and not processable by hot-melt coating. The aim of this study was to produce dense and abrasion-resistant granules with high drug content ( > 80%) via roller compaction for hot-melt coating process optimization. The selected API was ibuprofen sodium dihydrate, a salt of ibuprofen with improved...
25. June 2016
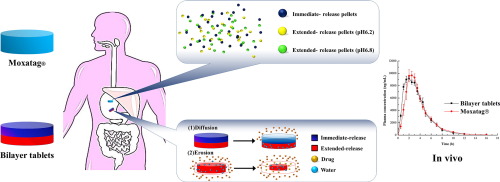
Abstract Multilayer/bilayer tablets have been applied for the formulation of incompatible components for compound preparations, but more often they are used to modify drug release. The objective of this study was to explore the feasibility of developing, using a bilayer tablet strategy, an immediate- and extended-release formulation of amoxicillin. The formulation of each layer was optimized separately and the bilayer tablets were compressed at an immediate/extended layer weight ratio of 3:7....