Abstract
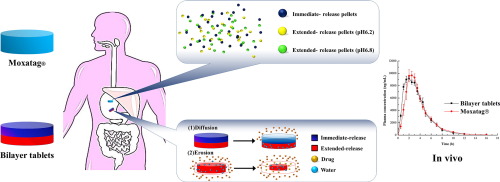
Multilayer/bilayer tablets have been applied for the formulation of incompatible components for compound preparations, but more often they are used to modify drug release. The objective of this study was to explore the feasibility of developing, using a bilayer tablet strategy, an immediate- and extended-release formulation of amoxicillin. The formulation of each layer was optimized separately and the bilayer tablets were compressed at an immediate/extended layer weight ratio of 3:7. The in vitro release of the bilayer tablets was evaluated and it was found to be very similar to Moxatag®, an immediate- and extended-release formulation approved by FDA. The kinetic mechanism study showed that the release of the bilayer tablets correlated better with the Ritger–Peppas model (correlation coefficient R = 0.9963) and a non-Fickian drug release mechanism, and its release was principally driven by diffusion and secondarily by polymer erosion. The stress testing demonstrated that high temperature and humidity are potential risk factors affecting the quality of the bilayer tablets. In addition, the bilayer tablets showed a similar bioavailability to Moxatag® in beagle dogs. In conclusion, the results of the present study demonstrated for the first time the feasibility of developing an immediate- and extended-release formulation of amoxicillin for once-daily use using a bilayer tablet strategy.