- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
15. September 2018
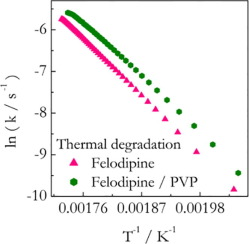
The present study explores the hypothesis that a polymer can affect the thermal stability of a drug in solid polymer-drug dispersions. The hypothesis is tested in a systematic fashion by combining isoconversional kinetic analysis with thermogravimetric measurements on several solid dispersions. Experimental systems involve three drugs: indomethacin (IMC), felodipine (FD), and nifedipine (ND) and their solid dispersions with polyvinylpyrrolidone (PVP). It is found that PVP stabilizes IMC but...
04. September 2018
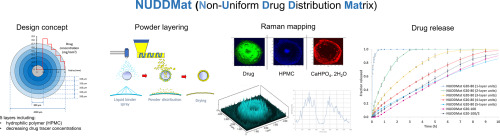
A decrease in the release rate over time is typically encountered when dealing with hydrophilic matrix systems for oral prolonged release due to progressive increase of the distance the drugmolecules have to cover to diffuse outwards and reduction of the area of the glassy matrix at the swelling front. In order to solve this issue, a novel formulation approach based on non-uniformdistribution of the active ingredient throughout the swellable polymer matrix was proposed and evaluated. Various...
28. May 2018
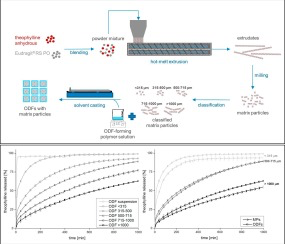
Orodispersible films (ODFs) are an advantageous dosage form to accomplish patient convenience and compliance in oral drug delivery. They provide a number of special application features, such as the ease of administration without water and suitability for patients with swallowing problems. However, this promising dosage form has been limited to immediate release formulations so far. The aim of this study was to develop a thin film produced by solvent casting, which is rapidly disintegrating...
23. January 2017
Abstract This study has investigated the potential use of zein protein from corn as a pharmaceutical excipient for formulation of oral controlled-release matrices. SMCC (silicified microcrystalline cellulose)-Prosolv® 90, SMCC HD-Prosolv® 90 HD, Avicel® PH 102 and Mannitol 60 were evaluated as co-excipients to improve the processability of zein powder. Zein/SMCC-Prosolv® 90 blend had the best flowability and tabletability. Tablets with tramadol hydrochloride, zein and SMCCProsolv® 90 were...
14. September 2016
Abstract This work investigates whether the solubility of poorly soluble compounds can be improved by using mesoporous magnesium carbonate (MMC) as the drug delivery system. A solvent evaporation method was used to load structurally diverse model drugs (celecoxib, cinnarizine and griseofulvin) into the pores of MMC. The drug-loaded carrier system was then characterized in terms of porosity, crystallinity, and release profiles by a variety of experimental techniques, including X-ray diffraction,...
09. August 2016
Abstract The aim of this study is to present the possibility of using of co-processed dry binders for formulation of matrix tablets with drug controlled release. Hydrophilic matrix tablets with tramadol hydrochloride, hypromellose and different co-processed dry binders were prepared by direct compression method. Hypromelloses Methocel™ K4M Premium CR or Methocel™ K100M Premium CR were used as controlled release agents and Prosolv® SMCC 90 or Disintequik™ MCC 25 were used as co-processed...
09. August 2016
Abstract This work aimed at developing enalapril maleate granules in order to improve its stability in solid dosage form. Granules were prepared by hot melt granulation using a fluidized bed apparatus. Gelucire 50/13®, polyethylene glycol 6000 e Poloxamer 407® were studied and compared as binders in 2 × 2 factorial designs where the proportions of enalapril maleate, binders and spray dried lactose were varied. The granulation process resulted in high yields and granule sizes that...
25. June 2016
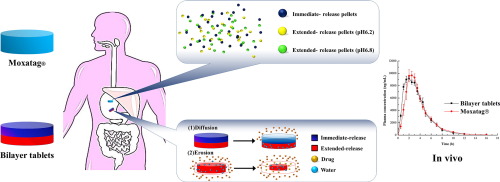
Abstract Multilayer/bilayer tablets have been applied for the formulation of incompatible components for compound preparations, but more often they are used to modify drug release. The objective of this study was to explore the feasibility of developing, using a bilayer tablet strategy, an immediate- and extended-release formulation of amoxicillin. The formulation of each layer was optimized separately and the bilayer tablets were compressed at an immediate/extended layer weight ratio of 3:7....
22. January 2016
Excipients used in the solid drug formulations differ in their NMR relaxation and 13C cross-polarization (CP) kinetics parameters.Therefore, experimental parameters like contact time of crosspolarizationand repetition time have a major impact on the registeredsolid state NMR spectra and in consequence on the results of the NMRanalysis. In this work the CP kinetics and relaxation of the most common pharmaceutical excipients: anhydrous α-lactose, α-lactose monohydrate, mannitol, sucrose,...
04. January 2016