- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
10. August 2018
Sticking and picking during tablet manufacture has received increasing interest recently, as it causes tablet defects, downtime in manufacturing, and yield losses. The capricious nature of the problem means that it can appear at any stage of the development cycle, even when it has been deemed as low risk by models, tests, and previous experience. In many cases, the problem manifests when transferring the process from one manufacturing site to another. Site transfers are more common now than in...
07. October 2017
In the context of continuous pharmaceutical oral dosage manufacturing, a control system is essential to ensure that the critical quality attributes (CQAs) are maintained within the regulatory constraints by mitigating variations generated in upstream operations. Such a system is essential to the Quality by Design (QbD) paradigm shift, which can ensure predefined end quality attributes are achieved within an optimal economic and time bracket. In this work, an advanced model predictive control...
16. September 2017
Abstract We aimed to understand the factors controlling mechanical particle coating using polymethacrylate. The relationship between coating performance and the characteristics of polymethacrylate powders was investigated. First, theophylline crystals were treated using a mechanical powder processor to obtain theophylline spheres (<100 μm). Second, five polymethacrylate latexes were powdered by spray freeze drying to produce colloidal agglomerates. Finally, mechanical particle coating was...
05. September 2017
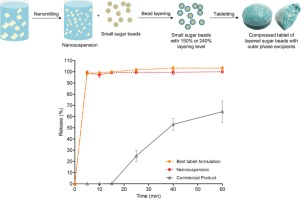
Abstract There has been limited research done on the downstream processing of nanosuspensions into solid oral dosage forms. This paper demonstrates the bead layering process with a layering level at 150% and 240%, as well as the selection and justification of the outer phase excipients for tabletability and disintegrating properties. In a previous study, an itraconazole nanosuspension stabilised by SDS and HPMC E5 was layered onto sugar beads with coating polymer HPMC VLV. In the current study,...
18. April 2017
Abstract There is more research required to broaden the knowledge on the downstream processing of nanosuspensions into solid oral dosage forms, especially for coated nanosuspensions onto beads as carriers. This study focuses on bead layering as one approach to solidify nanosuspensions. The aim was to systematically investigate the influence of type of coating polymer (HPMC VLV vs. copovidone), bead material and bead size (sugar vs. MCC, and small vs. large) and coating thickness (50%–150%...
10. April 2017
Abstract The improvements in healthcare systems and the advent of the precision medicine initiative have created the need to develop more innovative manufacturing methods for the delivery and production of individualized dosing and personalized treatments. In accordance with the changes observed in healthcare systems towards more innovative therapies, this paper presents dropwise additive manufacturing of pharmaceutical products (DAMPP) for small scale, distributed manufacturing of...
08. March 2017
Abstract There are several strategies for improving functional properties of starch-based materials. Blending with more hydrophobic compounds and bilayer formation are the most common methods. Barrier properties of several formulations obtained by different processing methods were measured. The properties of some obtained materials were compared with those usually employed in food packaging. The most promising materials were those starch PCL blends, compatibilized with grafted...
06. February 2017
Abstract The purpose of this work was to assess the impact of continuous mixing on tablet critical quality attributes (CQAs) manufactured using a continuous, direct compression process. A nine run design of experiments (DoE) that bracketed the range of commercially relevant mixer speeds, mixer orientations, and mass flow rates was executed using a formulation containing a cohesive drug substance at relatively low drug load. Drug substance dispensed concentration using loss-in-weight feeders was...
29. January 2017
Abstract The problem of gelation of concentrated protein solutions, which poses challenges for both downstream protein processing and liquid formulations of pharmaceutical proteins, is addressed herein by employing previously discovered viscosity-lowering bulky salts. Procainamide-HCl and the salt of camphor-10-sulfonic acid with l-arginine (CSA-Arg) greatly retard gelation upon heating and subsequent cooling of the model proteins gelatin and casein in water: Whereas in the absence of additives...
04. September 2016
Abstract Traditionally, the melt granulation for pharmaceutical products was performed at low temperature (<90°C) with high-shear granulators using low-melting waxy binders, and tablets produced using such granules were not amenable to large-scale manufacturing. The situation has changed in recent years by the use of twin screw extruder where the processing temperature could be increased to as high as 180°C and polymers with high Tg could be used as binders. In this study, different...