- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
21. September 2018
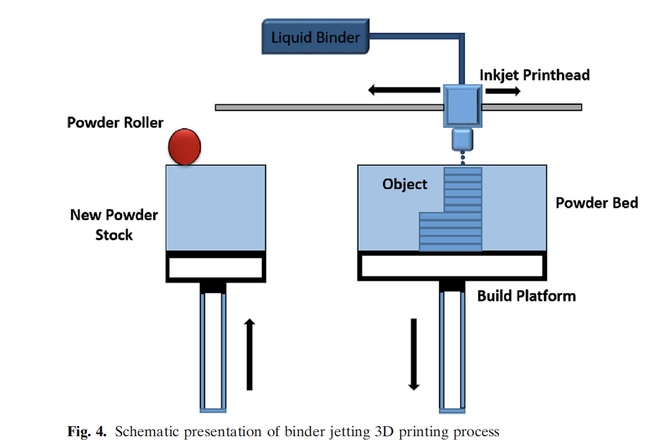
Three-dimensional (3D) printing was discovered in the 1980s, and many industries have embraced it, but the pharmaceutical industry is slow or reluctant to adopt it. Spiritam® is the first and only 3D-printed drug product approved by FDA in 2015. Since then, the FDA has not approved any 3D-printed drug product due to technical and regulatory issues. The 3D printing process cannot compete with well-established and understood conventional processes for making solid dosage forms. However,...
30. August 2018
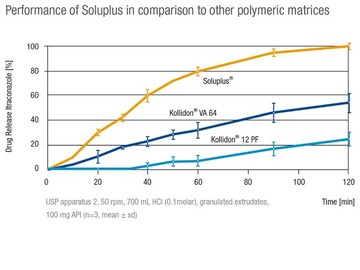
This study aimed to improve dissolution rate of valsartan in an acidic environment and consequently its oral bioavailability by solid dispersion formulation. Valsartan was selected as a model drug due to its low oral bioavailability (~23%) caused by poor solubility of this drug in the low pH region of gastrointestinal tract (GIT) and presence of absorption window in the upper part of GIT. Solid dispersions were prepared by solvent evaporation method with Eudragit® E100, Soluplus® or...
20. July 2018
Soluplus® has been approved in generics in Taiwan and Argentina and now filed in Brazil. Would like to be kept updated on Soluplus® regulatory status or need further Information? Sign up here!
19. July 2018
Did you know that Soluplus® is already approved in generics in Taiwan and Argentina and now filed in Brazil? Check the submentioned Soluplus® one pager with updated regulatory status per country. Soluplus® Key Customer Benefits Outstanding solubilization properties, especially for poorly soluble APIs Enables bioavailability enhancement Ideal for hot melt extrusion and all standard granulation techniques Market proven solution for unique formulation challenges Would like to be kept updated on...
14. May 2018
The objectives of this study were to explore sodium dodecyl sulfate (SDS) and Soluplus on the crystallization inhibition and dissolution of felodipine (FLDP) extrudates by bottom-up and top-down approaches. FLDP extrudates with Soluplus and/or SDS were prepared by hot melt extrusion (HME), and characterized by PLM, DSC and FT-IR. Results indicated that Soluplus inhibited FLDP crystallization and the whole amorphous solid dispersions (ASDs) were binary FLDP-Soluplus (1:3) and ternary...
25. April 2018
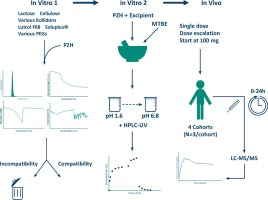
The anti-cancer drug pazopanib hydrochloride (PZH) has a very low aqueous solubility and a variable oral bioavailability. A new pharmaceutical formulation with an improved solubility may enhance the bioavailability and reduce the variability. A broad selection of polymer excipients was tested for their compatibility and solubilizing properties by conventional microscopic, thermal and spectrometric techniques. A wet milling and mixing technique was used to produce homogenous powder mixtures. The...
22. March 2018
Soluplus® is an innovative excipient that enables new levels of solubility, and bioavailability for poorly soluble active ingredients but still not monographed in any pharmacopoeia. An update on the current regulatory status can be found in the following pdf.file. More on Soluplus®
21. March 2018
In order to improve the aqueous solubility and dissolution of Tacrolimus (TAC), amorphous solid dispersions of TAC were prepared by hot melt extrusion with three hydrophilic polymers, Polyvinylpyrrolidone vinyl acetate (PVP VA64), Soluplus® and Hydroxypropyl Cellulose (HPC), at a drug loading of 10% w/w. Molecular modeling was used to determine the miscibility of the drug with the carrier polymers by calculating the Hansen Solubility Parameters. Powder X-ray diffraction and differential...
22. February 2018
Hot melt extrusion has gained considerable attention as a novel technique for taste masking of bitter APIs. The aim of this study was to investigate whether hot melt extrusion could be used to develop taste masked formulations of isoniazid and also to evaluate and correlate different taste assessment methods Two polymers with different physico-chemical properties, Soluplus and Eudragit E-PO were chosen as carriers for the drug. Eudragit E-PO has already been widely used for taste masking due to...
18. December 2017
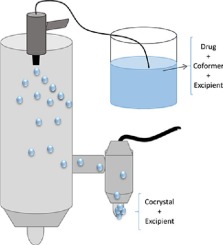
Spray drying is a well-established scale-up technique for the production of cocrystals. However, to the best of our knowledge, the effect of introducing a third component into the feed solution during the spray drying process has never been investigated.