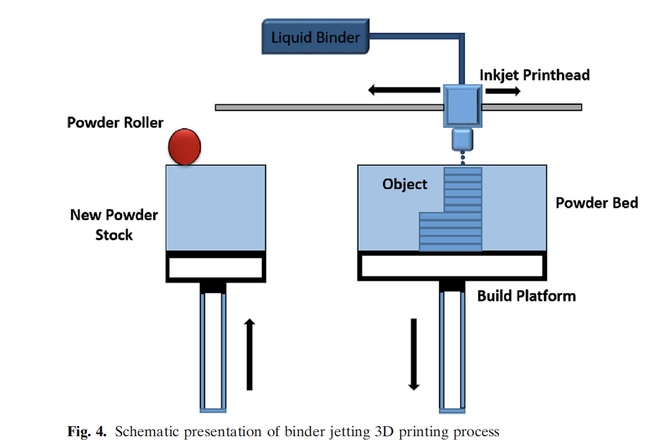
Three-dimensional (3D) printing was discovered in the 1980s, and many industries have embraced it, but the pharmaceutical industry is slow or reluctant to adopt it. Spiritam® is the first and only 3D-printed drug product approved by FDA in 2015. Since then, the FDA has not approved any 3D-printed drug product due to technical and regulatory issues. The 3D printing process cannot compete with well-established and understood conventional processes for making solid dosage forms. However, pharmaceutical companies can utilize it where mass production is not required; rather, consistency, precision, and accuracy in quality are paramount. There are many 3D printing technologies available, and not all of them are amenable to pharmaceutical manufacturing. Each 3D technology has certain prerequisites in terms of material that it can handle. Some of the pertinent technical and regulatory issues are as follows: Current Good Manufacturing Practice, in-process tests and process control, and cleaning validation. Other promising area of 3D printing use is printing medications for patients with special needs in a hospital and/or pharmacy setting with minimum regulatory oversight. This technology provides a novel opportunity for in-hospital compounding of necessary medicines to support patient-specific medications. However, aspects of the manufacturing challenges and quality control considerations associated with the varying formulation and processing methods need to be fully understood before 3D printing can emerge as a therapeutic tool. With these points in mind, this review paper focuses on 3D technologies amenable for pharmaceutical manufacturing, excipient requirement, process understanding, and technical and regulatory challenges.
There are many 3D printer technologies, but not all are amenable to pharmaceutical manufacturing. The technologies hold great promise for pharmaceutical manufacturing. Pharmaceutical manufacturers can adopt this technology to manufacture specialty products such as microparticles, implants, and intrauterine contraceptives, which require high precision and quality. However, there are technical and regulatory challenges that need to be addressed before pharma companies can fully embrace it. These include unavailability of cGMP compliant 3D printers, the importance
of understanding the variables associate with in-process and process controls, a need for the development of newer methods to assess quality of 3D products and cleaning validation, etc. Areas where 3D technologies require limited regulatory oversight are pharmacy and hospital setting where the prescription is compounded for special patient population (e.g., children, elderly or patients with special prescription requirements such as that associated with allergies to specific
excipient/dye components). Thus, there may be fewer hurdles to overcome when using 3D printing to generate tablets on an as-needed basis. However, the importance of recognizing the controls and challenges associated with 3D printing, and the need for assuring product quality and uniformity, remains critical to the future implementation of this approach. Thus, while 3D printing holds much promise for the future, as is hopefully seen through the points expanded upon in this review, this technology will need much work before it can be used to support patient care to meet the therapeutic objectives associated with personalized medicine.
Continue reading on Additive Manufacturing with 3D Printing: Progress from Bench to Bedside - a AAPS Journal 2018 article also describing "EXCIPIENTS AND DRUG PROPERTY REQUIREMENTS FOR 3D PRINTING PROCESSES"