- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
21. September 2018
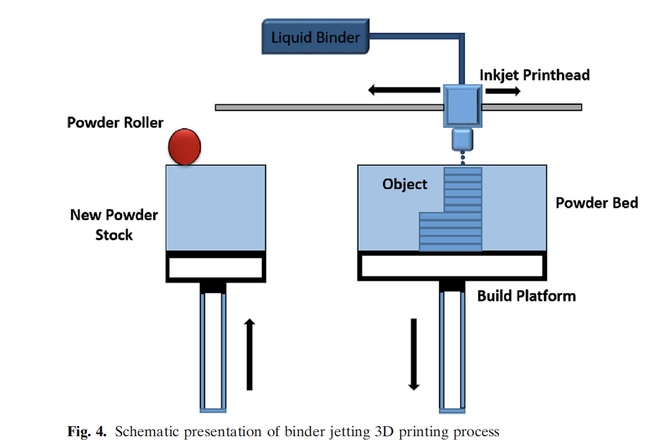
Three-dimensional (3D) printing was discovered in the 1980s, and many industries have embraced it, but the pharmaceutical industry is slow or reluctant to adopt it. Spiritam® is the first and only 3D-printed drug product approved by FDA in 2015. Since then, the FDA has not approved any 3D-printed drug product due to technical and regulatory issues. The 3D printing process cannot compete with well-established and understood conventional processes for making solid dosage forms. However,...
10. September 2018
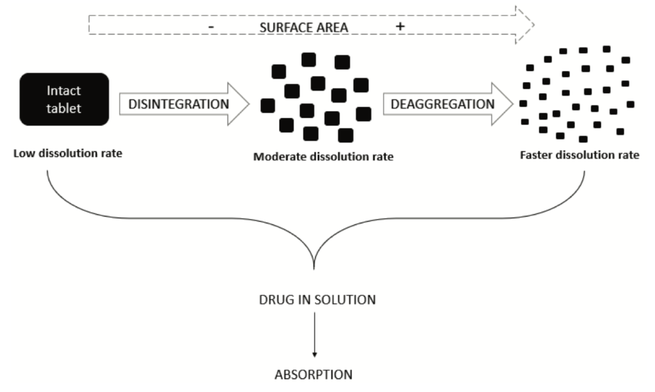
Disintegration is a physical process related to the mechanical breakdown of a tablet or granulate particle into smaller particles. This review investigates disintegration mechanisms, pharmacopeial use of the disintegration test and scientific studies showing its utility and potential as a pharmaceutical performance test. With a proper dosage form understanding and demonstration/justification of the mechanistic details of drug dissolution from a dosage form, dissolution testing might be replaced...
03. September 2018
Nowadays, the freeze-drying of liposome dispersions is still necessary to provide a solid dosage form intended for different routes of administration (i.e., parenteral, oral, nasal and/or pulmonary). However, after decades of studies the optimization of process conditions remains still challenging since the freezing and the dehydration destabilize the vesicle organization with the concomitant drug leakage. Starting from the thermal properties of phospholipids, this work reviews the main...
07. July 2018
“If formulation science is the Cinderella of drug development, then excipients are the Cinderellas of formulation science”. R. C. Moreton, Finn Brit Consulting This chapter assumes some familiarity on the part of the reader with formulation and excipients, but familiarity can breed contempt. Application of Quality by Design requires thinking beyond pharmacopoeial compliance and fixed formulae, to avoid the inevitable quality problems arising from the use of complex ingredients in complex...
17. May 2018
Current in vitro disintegration methods for polymeric films are qualitative and introduce significant user bias. The goal of these studies is to develop a novel, quantitative disintegration technique which can be used to characterize polymeric films in vitro. Methods A method was developed using a Texture Analyzer instrument to evaluate film disintegration. Solvent-casted, clinically advanced, anti-HIV, vaginal films as well as marketed vaginal films were used throughout these studies. Method...
20. February 2018
Purpose The FDA’s process validation guidance 2011 has rightly resulted in discontinuing the “one size fits all” practice. The guidance aligns process validation with quality by design and quality risk management guidelines. However, the process validation guidance has thrown a challenge with respect to determining the statistically appropriate number of batches for process performance qualification (PPQ) stage. This study reviews various approaches for estimating the number of PPQ...
11. January 2018
Accelerating trends in the pharmaceutical industry have made successful technology transfer more critical than ever. Mergers, acquisitions, the rise of generics, the closing of plants and the construction of new ones -- these trends and events typically entail the transfer of products and processes from one site to another. New products, too, must often be moved from the development site to a manufacturing site prior to launch.
25. November 2017
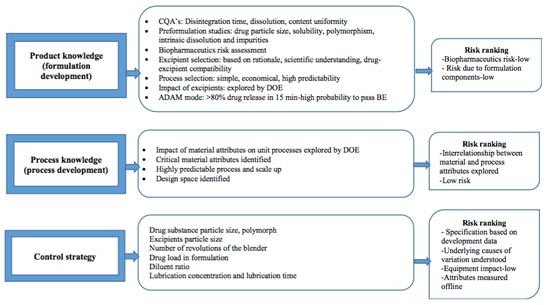
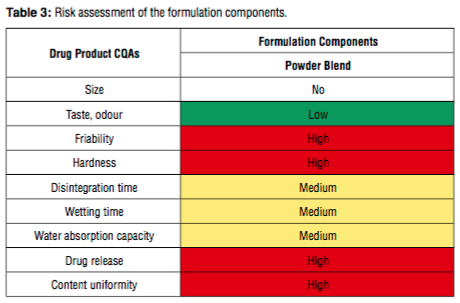
In this study, within the framework of Quality by Design which is a systemati- cally scientific approach which enables to understand and control the production and formulation variables during the process design and development, different parameters in the formulation and production process were detected and criti- cal process parameters and critical material attributes were determined via risk evaluation methods. Then, different oral disintegrating tablet formulations were prepared and tested b
20. November 2017
12 – 14 March 2018 - Berlin
Pharma-Excipients readers can register with a 25% discount off the current price.
05. November 2017
Poor water solubility of drugs fuels complex formulations and jeopardizes patient access to medication. Simplifying these complexities we systematically synthesized a library of 36 sterically demanding counterions and mapped the pharmaceutical design space for amorphous ionic liquid strategies for Selurampanel, a poorly water soluble drug used against migraine.