Purpose
The FDA’s process validation guidance 2011 has rightly resulted in discontinuing the “one size fits all” practice. The guidance aligns process validation with quality by design and quality risk management guidelines. However, the process validation guidance has thrown a challenge with respect to determining the statistically appropriate number of batches for process performance qualification (PPQ) stage. This study reviews various approaches for estimating the number of PPQ batches, and their merits and limitations. Additionally, a knowledge factor-based method in which residual risk level is related to the knowledge factor by a probability scale is proposed.
Methods and Results
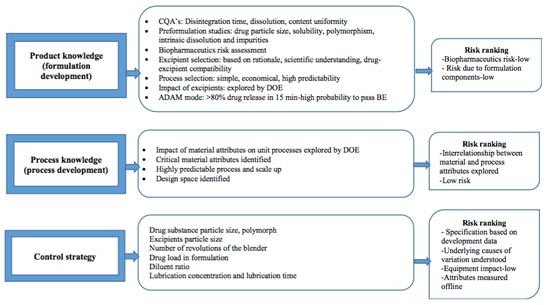
The risk-based methods assign a confidence level to unit processes based on the risk posed to critical quality attributes of the product. The level of product understanding and residual risk would determine the number of PPQ batches required for process validation. The knowledge factor-based method like other Bayesian methods provides an opportunity to incorporate knowledge gained during product/process development and scale up studies for estimating PPQ batch numbers. The number of batches required using this method are 6, 12, and 15, respectively, for low-, moderate-, and high-risk processes with corresponding knowledge factors of 0.1, 0.5, and 0.9.
Conclusions
Greater understanding and knowledge of product would reduce the requirement of PPQ batches remarkably. On the other hand, the higher residual risk level indicates knowledge gaps in product understanding; consequently, higher number of PPQ batches would be required to gain confidence in the product and the process before commercialization.