- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
28. March 2018
Mini-tablets with diameters of 2.0, 2.5, and 3.0 mm are coated in two different lab-scale fluidized bed coaters equipped with a Wurster draft tube. The main focus of the research is to evaluate the inter-particle coating variability, and to assess the contribution of cycle time variation. Cycle times are measured using a photoluminescent tracer with a detector mounted on the top of the draft tube. The number of passes variability is represented from 5 to 28% of the total coating variability....
27. September 2017
Because of the multiple roles they play in drug product formulations, excipient quality is as important as that of the API, so standards are needed to ensure their purity and reliability.
17. November 2016
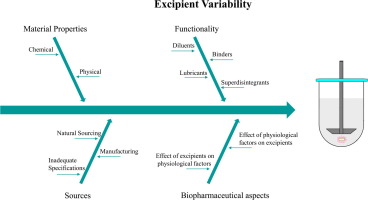
Abstract Implementation of Quality by Design approaches in pharmaceutical industry requires a sound understanding of the parameters triggering final product variability. Excipients, although generally regarded as inert components, are of great significance in terms of solid dosage form development and any variation in the material attributes may impact drug product performance. Sourcing, production and processing are contributing factors to excipient variability. Interchange between different...
26. September 2016
Abstract Dissolution from the pharmaceutical formulation is a prerequisite for complete and consistent absorption of any orally administered drug, including anticancer agents (oncolytics). Poor dissolution of an oncolytic can result in low oral bioavailability, high variability in blood concentrations and with that suboptimal or even failing therapy. This review discusses pharmaceutical formulation aspects and absorption pharmacokinetics of currently licensed orally administered oncolytics. In...
12. July 2016
The authors evaluated the performance and robustness of controlled-release tablets made with HPMC blends of unimodal and bimodal molecular weight distribution. Link to Article
07. December 2015
It is rare to have a pharmaceutical dosage form presented with just the pure active pharmaceutical ingredient because the drug substance does not possess adequately desirable physical attributes to be processed into the final dosage form. Consequently, additives or excipients which are inert ingredients serving a functional purpose are added to enhance the overall properties of the final formulation for ease of processability or drug product performance. More
19. October 2015
BY AJIT S. NARANG, MIKE TOBYN, AND ROBERT A. REED Excipient variability has been studied in areas such as particle characteristics, cross-linking, or reactive impurities with the goals of (a) identifying and quantitating potential variability in commonly used excipients, and (b) either designing formulations and processes that can accommodate expected variability or developing an understanding of the excipient variability design space and a control strategy for drug product and excipients....
09. October 2015
"Making mini-formulations of excipients in the lab and assessing their processability and stability cannot capture the full potential for variability. It’s better to map an API’s susceptibilities to potential degradation pathways, followed by a risk-based assessment that reveals an excipient’s impurity profile." More
21. September 2015
Excipients do cause batch failures. But we need to ask: How often are they the culprit and can we elucidate what role excipient variability plays in this manufacturing fact of life? More
29. July 2015
By: Joseph Kushner, IV, Ph.D.Senior Principal ScientistDrug Product DesignWorldwide Research & Development Pfizer Inc More