Abstract
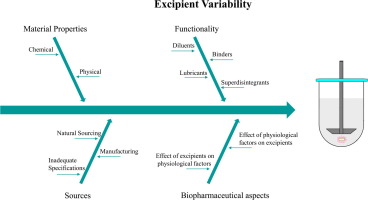
Implementation of Quality by Design approaches in pharmaceutical industry requires a sound understanding of the parameters triggering final product variability. Excipients, although generally regarded as inert components, are of great significance in terms of solid dosage form development and any variation in the material attributes may impact drug product performance. Sourcing, production and processing are contributing factors to excipient variability. Interchange between different suppliers can lead to final products with different quality attributes. Identification of excipient critical material attributes is not straightforward, as criticality must be linked to functionality and it is well recognized that the mechanisms by which excipients exert their action are not fully understood. Investigating the impact of excipient variability on in vitro dissolution could enable scientists to get an insight on the in vivo behavior of drug products and potentially tolerate variability. A thorough understanding of excipient material properties, product components interactions and the effect of the gastrointestinal tract heterogeneity on excipients and drug release is recommended. This review aims to present current knowledge on excipient critical material attributes and their link to biopharmaceutical behavior and dissolution characteristics. Attempts to describe the impact of physiological conditions on excipient functionality are also addressed. Excipient properties that are considered crucial to drug product performance in a biorelevant perspective are elucidated.