- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
10. November 2017
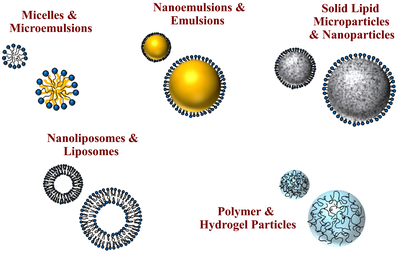
The design and development of nanoparticle- and microparticle-based delivery systems for the encapsulation, protection, and controlled release of active agents has grown considerably in the agrochemical, cosmetic, food, personal care, and pharmaceutical industries.
22. November 2016
Abstract Crospovidone is a commonly used tablet disintegrant. However, the synthetic disintegrant has been known to be hygroscopic and high moisture content in crospovidone used could exert deleterious effects on tablets formulated with it. The objective of this study was to elicit a better understanding between crospovidone-water interaction and its effect on disintegrant performance. Moisture sorption and desorption isotherms were obtained together with the enthalpy of immersion. Crospovidone...
20. September 2016
Abstract Formation of core-shell nanocomposites of Fenofibrate and Itraconazole, model poorly water soluble drugs, via fluidized bed (FB) coating of their well-stabilized high drug loaded nanosuspensions is investigated. Specifically, the extent of dissolution enhancement, when fine carrier particles (sub–50 μm) as opposed to the traditional large carrier particles (>300 μm) are used, is examined. This allows testing the hypothesis that greatly increased carrier surface area and more...
25. April 2016
Miniaturised solvent casting (MSC) has been developed as a method for screening the stability of amorphous solid dispersions (ASD) of BCS class II drugs. The aim of the work was to further develop a rapid screening technique for drug-polymer amorphous dispersions made by solvent removal techniques. A second aim was to assess the impact of varying dissolution solvent on the resultant ASD stability. The technique was rapid, repeatable and practically straightforward. Storage stability of...
30. March 2016
A pharmaceutical compound was used to study the effect of batch wet granulation process parameters in combination with the residual moisture content remaining after drying on granule and tablet quality attributes. The effect of three batch wet granulation process parameters was evaluated using a multivariate experimental design, with a novel constrained design space. Batches were characterised for moisture content, granule density, crushing strength, porosity, disintegration time and...
08. March 2016
In the pharmaceutical tablet film coating process, we clarified that a difference in exhaust air relative humidity can be used to detect differences in process parameters values, the relative humidity of exhaust air was different under different atmospheric air humidity conditions even though all setting values of the manufacturing process parameters were the same, and the water content of tablets was correlated with the exhaust air relative humidity. Based on this experimental data, the...
05. March 2016
This study explored the effect of nano-crystalline cellulose (NCC) on Meloxicam (MX) solid dispersion (SD) prepared by co-grinding technique compared to micro-crystalline cellulose (MCC) in presence of lactose. MX-tablets were prepared by direct compression of different co-ground SDs or physical mixtures. The solubility, dissolution, SEM and DSC of different preparations were studied. Flow-through cell apparatus (FTC) was used to study the dissolution of MX from tablets at pH 7.4. Generally,...
17. February 2016
This study evaluated the effects of changing the amount of added water for moisture activated dry granulation (MADG) on the resulting granule characteristics and tablet properties. Water soluble fillers and binders were pre-mixed in a granulator, and the binder was activated by a small amount of water to form granules. The amount of added water was defined as the percentage of granulation water by mass to charged powder. Granules made with 0.0%, 1.0%, 1.5%, 2.0%, 2.5%, 3.0%, and 5.0% (w/w)...
16. February 2016
Disintegrant is one of the most important components in a typical tablet dosage form. It is responsible for ensuring the break-up of the tablet matrix upon ingestion. Disintegrants act by different mechanisms, and a number of factors may affect their performance. It is important for formulators to understand how disintegrants function so as to be able to judiciously use disintegrants to develop optimized formulations. If the formulator is required to implement the quality by design paradigm...
02. February 2016
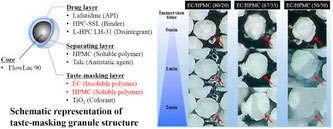
Taste-masking granules were prepared by coating granules with a taste-masking layer prepared by combining the water-insoluble and -soluble polymers, ethylcellulose and hypromellose, respectively. These granules showed an immediate drug release property after a suitable lag time. We confirmed that the dissolution behavior depended on the polymer ratio in the taste-masking layer. The result showed that the dissolution lag time and rate of the taste-masking granules were shortened and enhanced,...