Abstract
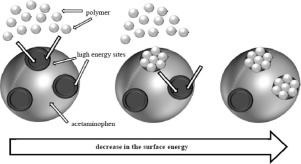
Many newly developed active pharmaceutical ingredients (APIs) have very low solubility in aqueous media. The preparation of solid dispersions (SDs) is one way of avoiding this problem. However, compound wettability and thus solubility are influenced by surface energy. In this study, we used inverse gas chromatography (IGC) to evaluate the surface energies of prepared SDs, and compared them with those obtained for physical mixtures (PMs). SDs containing different weight ratios of crystalline acetaminophen and one of three polymers (Kollidon® 12 PF, Kollidon® VA 64 or Soluplus®) were prepared by the melt-quenching of corresponding PMs. In all cases, as the polymer content increased, the surface energy decreased significantly. For the SDs and PMs containing Soluplus®, this decrease in surface energy showed the same non-linear trend. In the cases of Kollidon® 12 PF and Kollidon® VA 64, the trend was linear, with the SDs showing a steeper decrease in surface energy than the corresponding PMs. Typically, such decreases are ascribed to the dissolution of the crystalline structure of an API. Our results suggest that in the case of the Kollidons, the steeper decrease is caused by another mechanism, namely, strong API-Kollidon interaction leading to the less wettable surface of SDs.