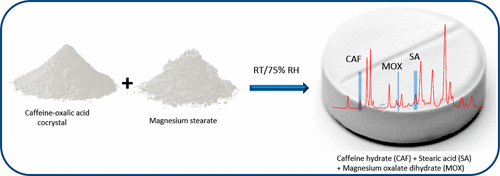
Caffeine–oxalic acid cocrystal, widely reported to be stable under high humidity, dissociated in the presence of numerous pharmaceutical excipients. In cocrystal–excipient binary systems, the water mediated dissociation reaction occurred under pharmaceutically relevant storage conditions. Powder X-ray diffractometry was used to identify the dissociated products obtained as a consequence of coformer–excipient interaction. The proposed cocrystal dissociation mechanism involved water sorption, dissolution of cocrystal and excipient in the sorbed water, proton transfer from oxalic acid to the excipient, and formation of metal salts and caffeine hydrate. In compressed tablets with magnesium stearate, the cocrystal dissociation was readily discerned from the appearance of peaks attributable to caffeine hydrate and stearic acid. Neutral excipients provide an avenue to circumvent the risk of water mediated cocrystal dissociation.
- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact