Abstract
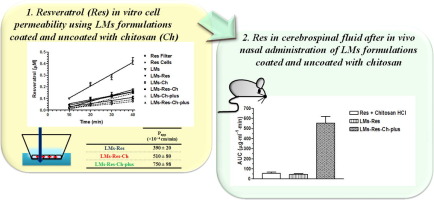
Lipid microparticles (LMs) uncoated or coated with chitosan and containing the neuroprotective polyphenol, resveratrol were developed for its targeting to the brain via nasal administration. The lipid microparticles loaded with resveratrol (LMs-Res) were produced by melt emulsification, using stearic acid as lipid material and phosphatidylcholine as the surfactant. The chitosan coated particles LMs-Res-Ch (1.75% w/v chitosan solution) and LMs-Res-Ch-plus (8.75 % w/v chitosan solution) were prepared by adding a chitosan solution to the formed particles. The mean diameter of the particles were 68.5±3.1 μm, 76.3±5.2 μm and 84.5±8.1 μm for LMs-Res, LMs-Res-Ch and LMs-Res-Ch-plus respectively, suitable for nasal delivery. Chitosan coating changed the particle surface charge from a negative zeta potential value (-12.7±2.1 mV) for the uncoated particles to a higher positive values respectively, 24.0±4.7 and 44.6±3.1 mV for the chitosan coated LM-Res-Ch and LM-Res-Ch-plus . Permeation studies across human NCM460 cell monolayers demonstrated that their transepithelial electrical resistance (TEER) values were not modified in the presence of free resveratrol, unloaded LMs, loaded LMs-Res or LMs-Res-Ch. On the other hand, the TEER values decreased from 150±7 to 41±3 Ω⋅cm2 in the presence of LMs-Res-Ch-plus, which corresponded to a significant increase in the apparent permeability (Papp) of resveratrol from 518±8×10−4 cm/min to 750±98×10−4 cm/min. In vivo studies demonstrated that no resveratrol was detected in the rat cerebrospinal fluid (CSF) after an intravenous infusion of the polyphenol. Conversely, the nasal delivery of resveratrol in a chitosan suspension or encapsulated in uncoated LMs-Res dispersed in water achieved the uptake of resveratrol in the CSF with Cmax after 60 min of 1.30±0.30 μg/ml and 0.79±0.15 μg/ml , respectively. However, a dramatic increase in the levels of resveratrol reaching the CSF was attained by the administration of an aqueous suspension of LMs-Res-Ch-plus with a Cmax after 60 min of 9.7 ± 1.9 μg/ml. This marked increase in the CSF bioavailability was achieved without any distribution in the systemic circulation, demonstrating a direct and specific nose to brain delivery.
Conclusions
Resveratrol-loaded LMs uncoated and coated with chitosan were developed in this study and evaluated in vitro and in vivo as a carrier system to enhance the targeting of resveratrol to the brain via nasal administration. To the best of our knowledge, the effect of microparticulate powder carrier on the in vivo nose to CSF uptake of resveratrol has not been reported before. Advantages of the LMs system described here include biodegradability of their components, specific brain delivery, ease of administration as well as non-invasiveness. Pharmacokinetic studies indicated that the intranasal administration of the LMs-Res-Ch-plus particles produced a marked increase in the bioavailability of resveratrol in the CSF which should enhance its neuroprotective effect for the treatment of neurological disorders.