Impregnation of active pharmaceutical ingredients (APIs) onto porous excipients has numerous benefits for solid dosage formulations. Previous work has successfully demonstrated the manufacturing of pharmaceuticals using fluidized bed (FB) impregnation of APIs onto porous carriers and discussed its advantages (such as easy to implement, improvement of blend uniformity and dissolution kinetics, and stabilization of amorphous APIs). This study aims to develop methods for analysis of the spatial distribution of the impregnated API inside the porous excipient. An understanding of the spatial distribution of the API can be important if one wants to achieve high drug loadings. In addition, the spatial distribution of the API can impact its dissolution rate. The impregnation profile is analyzed using energy dispersive X-ray spectroscopy (EDS). Two formulations are investigated using Fenofibrate and Acetaminophen (model APIs), impregnated onto Neusilin (porous excipient). Several methods are presented for particle embedding and cutting in order to produce cross-sections for analysis. Embedding with carbon-based resins/adhesives produces cross-sections with high quality but the resins contaminate the sample with carbon and reduce the detection of trace elements. Manually cutting particles immobilized on carbon tape or inorganic-based adhesives produces cross-sections with a higher degree of roughness but improves the detection of trace elements and reduces/eliminates carbon contamination in the sample, allowing for API detection by its carbon footprint. EDS analytical results showed that for both Fenofibrate and Acetaminophen formulations examined in this work, the API profile is highly uniform (detected by both carbon and characteristic trace elements).
Conclusions
In this article, we presented a method for cross-sectional analysis of impregnated excipient particles using energy dispersive X-ray spectros- copy. The aim of the work was to determine the methodology most suited for studying the impregnation profiles achieved during fluidized bed impregnation of APIs onto porous excipients. The focus of the anal- ysis was to develop methods for determining the relative spatial distri- bution of the active ingredient within the impregnated particle rather than a fully quantitative analysis. The APIs chosen for the investigational work were Fenofibrate and Acetaminophen and the porous carrier was Neusilin. Two other candidates were introduced (KAc and KI) as poten- tial surrogate substrates for impregnation studies.
Several particle embedding methods were demonstrated, highlight- ing their benefits and shortfalls. Acrylic resins (LR White) used for par- ticle immobilization showed the ability to produce excellent cross- sections with a smooth surfaces. In the case of Fenofibrate, the EDS anal- ysis of LR White embedded samples failed to produce the real API distri- bution due to the API's high solubility in the resin. In other cases (Acetaminophen and KI) the resin failed to polymerize due to a sub- strate/resin interaction. When KAc was embedded in LR White resin, the EDS analysis showed its full potential and ability to detect differ- ences in the impregnation distributions (uniform vs. egg-shell/egg-
yolk profiles). Cyanoacrylate adhesives (Super Glue) showed similar ability to produce high quality cross-sections without the drawback of a substrate/media interaction (lack of polymerization). A major disad- vantage of using embedding media was its penetration within the po- rous particle during sample preparation.
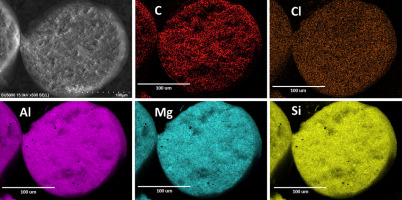
In order to eliminate resin penetration, a different method for pro- ducing cross-sections of particles was introduced. This involved the im- mobilization of a particle monolayer onto carbon tape or non-carbon, silicate-based Ni paste, followed by manually cutting the particles. This technique produced cross-sections with a higher degree of rough- ness but without any embedding media inside or around the particles. This improved the detection of trace elements from the API's molecule since glue did not penetrate the sample. In addition, the carbon in the sample from sources other than the API was significantly reduced, allowing its use for API detection.
Our results showed that EDS analysis based on both carbon and trace elements (Cl for Fenofibrate and N for Acetaminophen) showed a uni- form profile in both fluidized bed impregnation formulations. As far as we know, this is the first time the type of API profiles obtained in FB im- pregnation have been reported, underlying once more the potential of the impregnation process to produce highly desirable uniform distribu- tions. It is anticipated that the type of the API profile will be a function of the processing conditions and material parameters. These results are for a limited set of processing conditions and only for two APIs. Further work is needed to examine the robustness of these results. It would be expected that different processing conditions could lead to egg-shell or egg-yolk distributions of APIs. In addition, the porosity of the carrier is also expected to affect the distribution of API. Further experiments and studies should be carried out in order to determine how processing conditions and material properties affect the final API profile during FB impregnation.