Are you a pharmaceutical raw material producer and would like to optimize the handling of your quality documents?
Be ahead of the curve
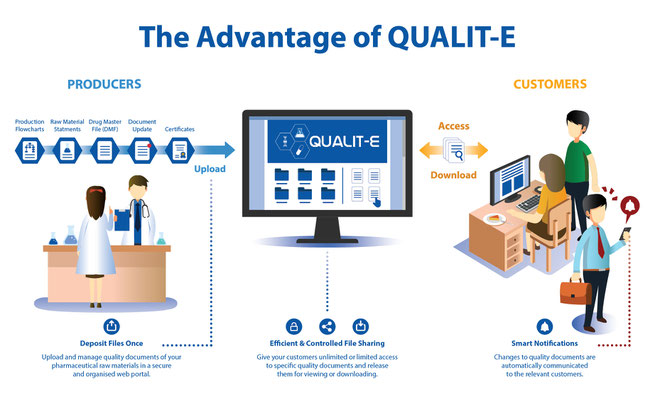
As a pharmaceutical raw material producer, sending several high-level quality statements and certificates daily is an essential part of the sales process. What if there were a simpler way? What if a secure, digital platform could enable you to upload quality documents only once and give authorizations to selected customers so they could download those documents and other sensitive data? Soon, Qualit-e Cloud will make this all possible.
Exchange files efficiently and safely
This unique software allows you to quickly exchange important statements and certificates without countless emails. If there is a change notification, only relevant customers will be automatically notified. You will also have the option to authorize access to a distributor, who can then alert that authorization to their customers.
Our intuitive, intelligently designed web portal is coming soon
Dramatically reduce the workload of your quality, regulatory and sales team. Manage change control information and notify customers of new file versions. Your customers will appreciate the improved, streamlined process. Best of all, you’ll save time and money.
You and your customers deserve Qualit-e Cloud. Sign up today to be the first to know when we go live. More information & registration
Participate our survey: Reduce the workload of your quality and regulatory team with Qualit-e Cloud - the new webportal for sensitive pharma quality documents.