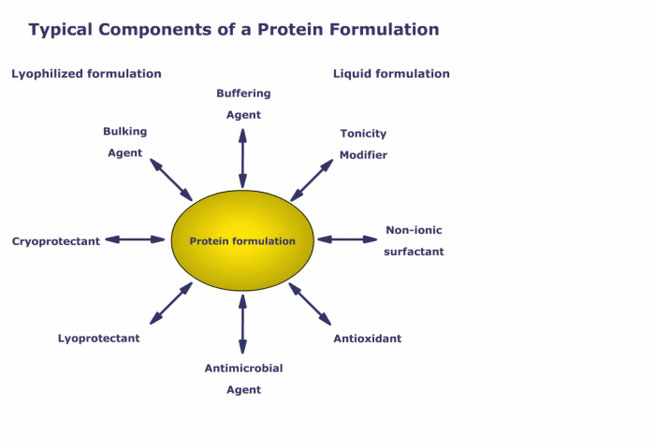
Preservatives are excipients essentially needed in pharmaceutical multi-dose formulations to prevent microbial growth. Among available substances, phenol is widely used for parenterals, however, it is known to interact with non-ionic surfactants like polysorbate and potentially with the active pharmaceutical ingredient.
Although the need for combinations of surfactants and preservatives is growing, to date possible molecular interactions which can eventually weaken the stability and antimicrobial activity of the formulation are not yet well understood and properly investigated.
In the current study, the binding of phenol to a model fusion protein as well as to polysorbate 20 was investigated. For this purpose, the fraction of bound phenol was successfully quantified via diffusion ordered NMR spectroscopy.
The binding of phenol to the surfactant is negligible in pharmaceutically relevant polysorbate concentrations, but the binding to the employed active pharmaceutical ingredient was relevant and concentration dependent. The resulting consequence of this interaction was the decrease of the antimicrobial efficacy.
As a final outcome of this study, NMR analysis is proposed as a material saving method to be used in combination with the antimicrobial activity testing described in the Pharmacopoeias.