Gelucire® is Gattefossé’s brand of semi-solid excipients originally designed for hard gelatin capsule molding. The name results from the fusion between « gélule » meaning capsule and « cire » meaning wax. Following the name, there is a set of two figures: the first (eg 44) indicates the melting point in Celsius degree, the second (eg 14) indicates the theoretical HLB (Hydrophilic Lipophilic balance).
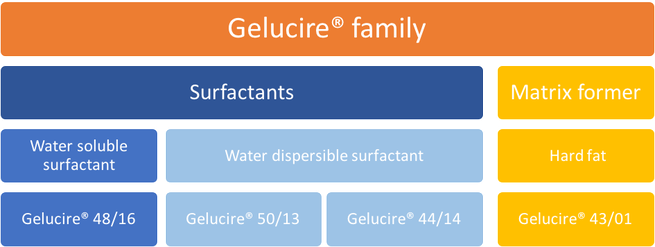
The family is composed of four products with their own characteristics, specificities and functionalities:
- One water soluble surfactant: Gelucire® 48/16
- Two water dispersible surfactants: Gelucire® 44/14 and Gelucire® 50/13
- One matrix former: Gelucire®43/01
Other grades existed in the past but are no longer available.
I. Surfactants
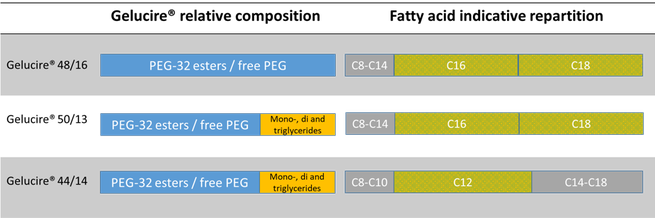
Gelucire® surfactants result from an esterification reaction between a polar moiety (PEG 32, molecular weight 1500) and an apolar moiety (vegetable oils or fatty acids). The final product is either a pure PEG ester (Gelucire® 48/16) or a mixture of PEG esters and mono-, di- and triglycerides (Gelucire® 44/14 and Gelucire® 50/13). The fatty acid repartition depends on the original raw material: Gelucire® 48/16 and Gelucire® 50/13 are composed mainly of stearic and palmitic acids, whereas lauric acid is the main fatty acid of Gelucire® 44/14.
Surfactants are used in lipid based-formulations (LBF) to increase solubility and oral bioavailability of poorly water-soluble drugs.
1. Water soluble surfactant
Composed of PEG esters, Gelucire® 48/16 does not contain a glyceride fraction. It forms transparent micellar solution in aqueous media.
Gelucire® 48/16 is a polyethylene glycol monostearate (type I) NF and consists of PEG-32 (MW 1500) esters of palmitic (C16) and stearic (C18) acids. It is a solid at ambient temperature making it suitable for capsule filling, melt granulation and extrusion. It is available in pellet form.
An increase in solubility and in vitro oral bioavailability has been demonstrated with several model drugs: piroxicam, nifedipine, curcumin, ticagrelor and telmisartan. (access our publications on Gelucire®48/16).
2. Water dispersible surfactants
Composed of PEG esters and glycerides, these Gelucire® form a fine dispersion in water. They are self-emulsifying excipients.
Gelucire® 44/14
With complete characterization, numerous publications with in vivo and in vitro results and worldwide precedence of use including FDA IID listing, Gelucire 44/14 is versatile and suitable for many formulation processes: capsule molding, melt granulation and spray cooling.
Gelucire® 44/14 is a lauroyl polyoxyl/macrogol 32 glycerides NF/EP and consists of a small fraction of mono, di- and triglycerides and mainly PEG-32 (MW 1500) mono- and diesters of lauric acid (C12). Being derived from lauric acid, it is more hydrophilic than Gelucire® 50/13 (derived from palmitic and stearic acid) and will hydrate very easily. It is a semi-solid excipient, available as a block.
Gelucire® 50/13
Gelucire® 50/13 is a well characterized excipient, supported by numerous publications and worldwide precedence of use including FDA IID listing. It has similar surfactive properties to Gelucire® 44/14. However with a higher melting point and longer fatty acid chains it can have a release retarding effect when used at high concentration.
Gelucire® 50/13 is a stearoyl polyoxyl/macrogol 32 glycerides NF/EP and consists of mono, di- and triglycerides and PEG-32 (MW 1500) mono- and diesters of palmitic (C16) and stearic (C18) acids. It is available in pellet form.
II. Matrix former
Including an API in a glyceride matrix is a simple and efficient method for dispersion and protection from environmental conditions that affect stability, such as light, oxygen, pH and/or water.
Gelucire® 43/01
It is composed of glycerides only. Due to its low melting point (42-46°C), Gelucire® 43/01 is ideal for API protection and capsule filling. It has precedence of use.
Regulatory speaking, it is a hard fat EP/NF/JPE. Chemically speaking it is composed of mono-, di- and triglyceride esters of fatty acids (C8 to C18), the triester fraction being predominant. It is available in pellet form.
For more technical documentation and sample ordering: www.gattefosse.com