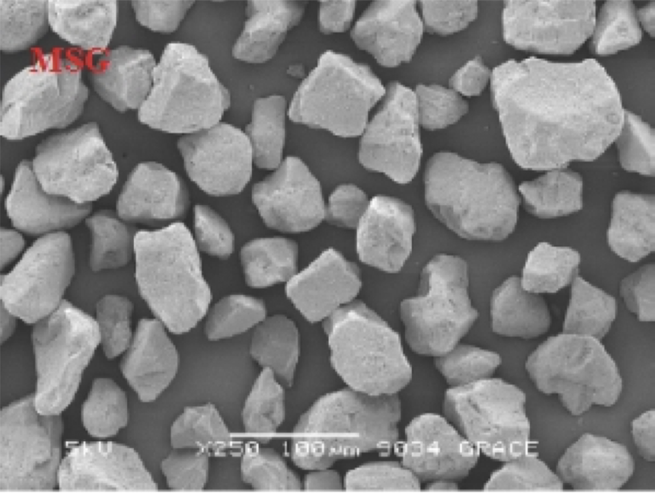
Conversion of liquid and semisolid lipids into free flowing powders is an advantageous technique, as the carriers display high surface area, strong adsorption capacity, ease of processing, and ability to generate lipid loaded free flowing powders which can be converted to solid dosage forms like tablets and capsules. A combination of density, adsorption capacity and desorption is found to be of importance in the selection of the right adsorbent. Adsorbents like magnesium aluminium silicates (MAS), granulated fumed silica (GFS) and mesoporous silica gel (MSG) were characterized by flow property measurements, particle size, scanning electron microscopy (SEM) and pore structure by mercury (Hg) intrusion study. SEM results reveal adsorbent morphology, whereas an intrusion-extrusion study reveal pore size distributions. Tablets and capsules of oil loaded adsorbents were prepared by conventional methods. Oil loaded adsorbents were evaluated for the ability to convert oil into powder, easy of processing into tablets and capsules, and release of the loaded oil (Vitamin E) or active (Glyburide). All adsorbents possess good flow property while MSG has higher density than GFS and MAS. This helps to deliver maximum active per unit volume. A wider pore size distribution of MAS was observed in comparison to MSG and GFS. MAS exhibited poor oil release from powder and its formulations, whereas GFS demonstrated closely similar release to MSG. Maximum 70% oil loaded MSG can be delivered in tablet dosage form andMSG can deliver the highest amount in limited volume capsules due to its high density. Hence, lower density and poor oil release from MAS limit its applications in solid oral drug delivery, while both MSG and GFS proved to be suitable.
Conclusion
All three solid porous carriers studied are found to have adequate flow properties. As opposed to the other carri- ers, the flow properties of MSG and oil loaded MSG do not change significantly, and MSG has a higher bulk den- sity allowing for easy processing and the ability to de- liver maximum amounts of liquid in a given volume such as a capsule (volumetric adsorptive capacity). All carriers found to be acceptable (average 1 carrier:3 oil) in the oil adsorption study (Table 4); oil desorption however was observed to differ between carriers with oil desorption of MSG=GFS>MAS. According to the Glyburide release study, MSG is able to carry SMEDDS formulations adequately and more effectively. Oil loaded MSG can be formulated in both tablets and capsules. In conventional DC tablets, a max- imum of 40% can be delivered while up to 70% can be formulated using PVP ethanol dispersion as a binder. In terms of oil release from a tablet, MSG=GFS>MAS. In cap- sules, the highest amount of oil loaded MSG can be deliv- ered as compared to MAS and GFS and also MSG demon- strated maximum oil release compared to GFS and MAS. Also, both MSG and oil loaded MSG is proven to be com- patible with gelatin and Vcaps® HPMC capsules. It can be concluded that highly porous silicate carriers are an ade- quate choice for pharmaceutical formulators in delivery of oil and lipid based formulations. Ongoing investigations are required to understand the molecular interactions that may occur with the adsorbent and to understand how GIT enzymes may play a role in driving desorption.
De Gruyter Open access: https://doi.org/10.2478/mesbi-2014-0004