The material residence time distribution in a continuous manufacturing process can be utilized to develop, design and justify the process control strategy. This paper successfully demonstrates using both major and minor formulation component step changes to determine the system response using either Near Infrared Spectroscopy or process parameters. These options provide development flexibility to determine the system’s material residence time earlier in the development process and more cost effectively.
Conclusions:
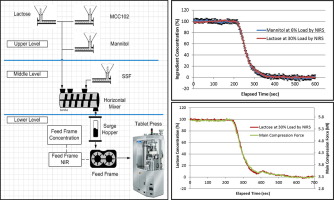
Minor and major ingredient step changes produced similar response profiles using the NIR application located in the tablet press feed frame for this unit formula. This result enables using major/minor ingredients to characterize the system RTD when the unit formula drug substance cannot be detected by the NIR application or when the drug substance supply is very rare in early-phased pharmaceutical product development.
The NIR application, tablet press pre-compression and tablet press main compression force parameters produce similar response curves when performing the major ingredient step changes for this unit formula. These results enable using process parameters in-lieu of an NIR application to produce system response curves that are inputs to the flow sheet model to determine the system’s RTD saving both time and money.