- Home
- Blog
- News
- Basics
- Sources
- Agencies, Regulatory & Organisations
- CERSI Excipients Browser
- Excipient Report
- Excipient DMF List
- EXCiPACT Certified Companies
- Excipient Documentation
- Excipient EINECS Numbers
- Excipient E-Numbers
- FDA Inactive Ingredient List
- FDA GRAS Substances (SCOGS) Database
- IPEC Americas
- USP - U.S. Pharmacopeia
- Definitions
- Whitepapers / Publications
- Supplier
- Services
- Media
- Events
- 1st pharmaexcipients Poster Award
- Event Calendar
- Events featured by pharma-excipients
- 4th Annual Formulation & Drug Delivery Congress
- DDF Summit
- ExcipientFest Americas
- ExcipientFest Asia
- Global CompliancePanel
- International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems
- Formulation & Drug Delivery USA Congress
- Laboratory Medicine 2018
- Making Pharmaceuticals Europe
- Making Pharmaceuticals Exhibition
- Pharma Integrates
- PharmaExcipients China @CPhI China
- TTC Technology Training Center
- Jobs
- Online Sourcing
- Contact
07. September 2018
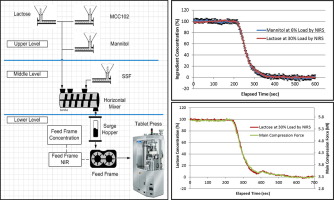
The material residence time distribution in a continuous manufacturing process can be utilized to develop, design and justify the process control strategy. This paper successfully demonstrates using both major and minor formulation component step changes to determine the system response using either Near Infrared Spectroscopy or process parameters. These options provide development flexibility to determine the system’s material residence time earlier in the development process and more cost...
01. July 2018
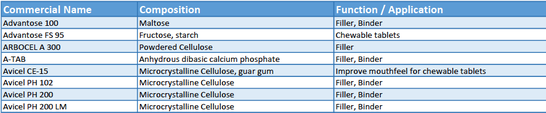
With the current hot topic of continuous manufacturing the topic of direct compression is also in the focus. There is a wide range of excipients for direct compression with different functions available in the market. pharmaexcipients.com has put together an initial overview as a list of products available for download and commenting.
10. April 2018
The pharmaceutical industry has historically taken a cautious approach to new advancements. But now, it is embracing the possibilities of continuous manufacturing technologies. While traditional batch based processes are still the industry standard, there are several products on the market that have utilized continuous manufacturing practices in their development. Over the last decade, this approach has generated a considerable amount of attention. To take a deeper look at the issues...
05. April 2018
The dehydration of biopharmaceutical products through drying provides numerous benefits, including ease of handling and storage, reduction in transportation costs, and improved stability. Typically, the drying of biotherapeutics is accomplished through freeze-drying, however, the removal of water by lyophilization possesses several drawbacks, including lengthy drying times, low energy efficiency, and the high cost of purchasing and maintaining the equipment. Furthermore, freeze- drying is a...
31. March 2018
Just found this chapter of the 2nd Edition of "Pharmaceutical Extrusion Technology" which can be of interest in the field of continuous manufacturing. Hot melt extrusion (HME) is a continuous process operation that has been successfully applied to the manufacture of quality drug products over the last decade [1–4]. 54HME achieves the molecular mixing of active pharmaceutical ingredients (API) and excipients at temperatures above their glass transition temperatures (T g) and/or melting...
16. March 2018
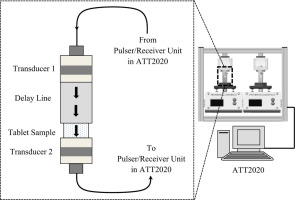
Currently, the compressed tablet and its oral administration is the most popular drug delivery modality in medicine. The accurate porosity and tensile strength characterization of a tablet design is vital for predicting its performance such as disintegration, dissolution, and drug-release efficiency upon administration as well as ensuring its mechanical integrity.
20. February 2018
As the pharmaceutical industry increasingly adopts continuous manufacturing technology, significant attention must be paid to process analytical technology (PAT), process integration, and process control. Published information is no substitute for hands-on comprehensive training, which is critical to implementing and operating continuous pharmaceutical manufacturing systems effectively and efficiently. In this article, an intensive hands-on training course has been developed and implemented at...
08. February 2018
In the highly regulated pharmaceutical industry, manufacturers can’t help but wonder what regulatory authorities like the FDA think of emerging technologies. So what do the global authorities have to say about continuous manufacturing?
30. January 2018
Continuous Manufacturing Technology continues to gain importance in pharmaceutical manufacturing.
Although traditional processes like direct compression, roller compaction, or wet granulation are used within the continuous lines, the requirement for ingredients may differ from traditional batch processing.
30. December 2017
The objective of this study was to devise robust and stable continuous manufacturing process settings, by exploring the design space after an investigation of the lubrication-based parameters influencing the continuous direct compression tableting of high dose paracetamol tablets.