Abstract
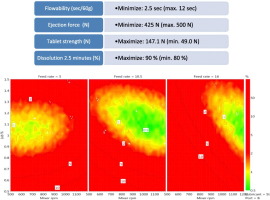
The objective of this study was to devise robust and stable continuous manufacturing process settings, by exploring the design space after an investigation of the lubrication-based parameters influencing the continuous direct compression tableting of high dose paracetamol tablets. Experimental design was used to generate a structured study plan which involved 19 runs. The formulation variables studied were the type of lubricant (magnesium stearate or stearic acid) and its concentration (0.5, 1.0 and 1.5%). Process variables were total production feed rate (5, 10.5 and 16 kg/h), mixer speed rpm (500, 850 and 1200 rpm), and mixer inlet port for lubricant (A or B). The continuous direct compression tableting line consisted of loss-in-weight feeders, a continuous mixer and a tablet press. The Quality Target Product Profile (QTPP) was defined for the final product, as the flowability of powder blends (2.5 s), tablet strength (147 N), dissolution in 2.5 min (90%) and ejection force (425 N). A design space was identified which fulfilled all the requirements of QTPP. The type and concentration of lubricant exerted the greatest influence on the design space. For example, stearic acid increased the tablet strength. Interestingly, the studied process parameters had only a very minor effect on the quality of the final product and the design space. It is concluded that the continuous direct compression tableting process itself is insensitive and can cope with changes in lubrication, whereas formulation parameters exert a major influence on the end product quality.
Conclusions
The aim was to determine robust and stable continuous manufacturing process settings, by devising a design space based on the investigation of lubrication dependent attributes which would influence continuous direct compression tableting of high dose paracetamol tablets. The obtained data verifies that a continuous manufacturing process is feasible for high dose direct compressible paracetamol tablets. The material properties of the main components have the most important influence on the process performance of a continuous manufacturing process. In this current study, the accuracy of feed rate of pre-blends was excellent. Interestingly, a high variation in the feed rates of lubricants did not exert a negative effect on the final product quality. It can be concluded that high variation in feed rates of lubricants could be eliminated with the good flow rate of main components and efficient blending unit operation. The type and concentration of lubricant had the greatest statistically significant effect on the studied responses. Stearic acid was better than magnesium stearate. The selection of lubricant type and its concentration have an effect on the dissolution profile only at the beginning of the dissolution (at the dissolution time point of 2.5 minutes), the ejection force, tablet strength and flowability. It can be concluded that continuous direct compression tableting process is rather insensitive and robust with respect to variations in lubrication. The process parameters have only a slight influence on the tablet properties. Adjusting the total feed rate and selection of inlet port might alter the flowability properties and maximum ejection force. Based on the tableting results, even a high variation in the feed rate of lubricants had no influence on the tableting process or the quality of the final product. However, the process parameters did not affect the quality of the tablet properties; in contrast, formulation parameters did exert a major influence on the properties of the end product. The created design space showed that this type of continuous manufacturing process line is suitable for producing high dose direct compressible paracetamol tablet which possessed a predetermined acceptable quality. However, in the future, longer runs should be completed to observe whether the quality of the product can be preserved as a function of time.
Recommended for you
- Developing a quality by design approach to model tablet dissolution testing: an industrial case study
- Film Coating Excellence by Biogrund
- Excipient DMF 3rd Quarter 2017
Don't miss any blog article - Sign up for the weekly blog digest!