Abstract
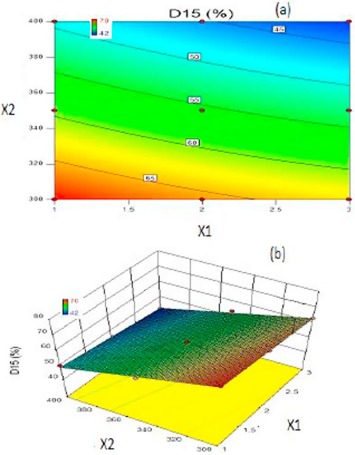
The work is based on pulsatile principles to deliver a programmed dose of Irbesartan, an angiotensin-II receptor antagonist for chronotherapy of hypertension induced by excessive secretion of aldosterone, thereby lower the blood pressure at early morning. Solid dispersion of Irbesartan, a BCS class II drug, was prepared by using Poloxamer-188 by melt method in ratio of 1:1 to increase the dissolution properties of drug. Compressed coated pulsatile tablets included a core layer consisting of Kyron T-134 as a super-disintegrant and pulsatile layer comprising of HPMC K4M and Eudragit RLPO. The prepared core tablets were evaluated for weight variation, hardness, thickness, friability, drug content, disintegration time and In vitro dissolution studies. Final core tablet (C8) was selected on the basis of disintegration time (23.33 ± 2.08 s). For optimization Face centred central composite design was employed to study the effect of independent variables viz. Weight ratio of HPMC K4M: Eudragit RLPO (X1) and Total weight of coating (X2) on dependent variables viz. Drug release lag time (Y1) and Drug release after lag time within 15 min (D15) (Y2). Results revealed positive influence of independent factors on responses. The data were statistically analyzed using ANOVA and were found to be statistically significant (P < .05). Mathematical modeling for kinetic studies revealed that the release profile after lag time followed first order kinetics. Accelerated stability studies for one month at 40 ± 2 °C/75 ± 5% RH showed no remarkable changes concluding that a successful pulsatile drug delivery system of Irbesartan was developed.